CBSE Class 12-science Answered
How density of unit cell is same as density of a substance?
Explain in detail with the help of an example.
Asked by Shubhi Gupta | 19 Apr, 2015, 08:08: PM
Dear shyamageeta@yahoo.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
We have not understood the query that you have posted. We would request you to clarify / provide additional details so that we may answer this to the best of the ability.
However, you can refer to the following explanation/solution for the query you have asked,
Unit cell is the smallest group of atoms which has the overall symmetry of a substance (crystal), and from which the entire lattice can be built up by repetition in three dimensions.
For example unit cell of sodium chloride crystal has the overall symmetry of a crystal, and from which the entire lattice can be built up by repetition in three dimensions.
Hence, the density of unit cell is same as density of a substance which is given by the formula,
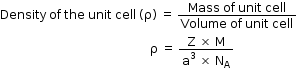
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Hanisha Vyas | 20 Apr, 2015, 12:32: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 07:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 09:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




