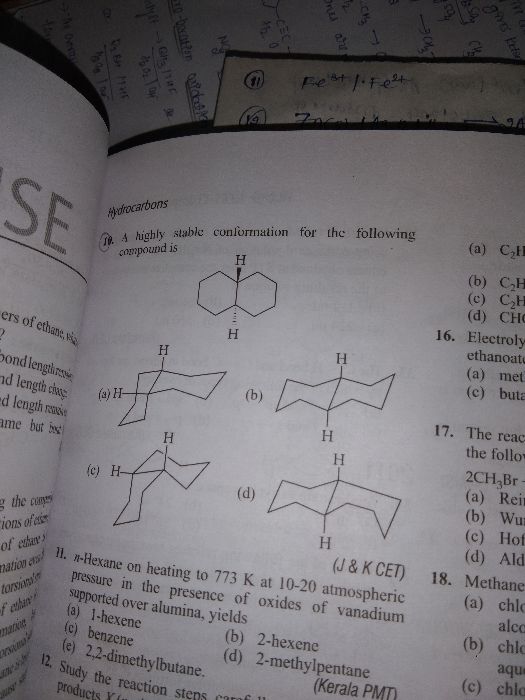
CBSE Class 11-science Answered
hello sir, I wanted to know, that why does aromatic compounds are more stable than alkanes ?
Asked by Kalyanrao Chavan | 29 Sep, 2013, 10:17: PM
Aromatic compounds are more stable than alkanes because of resonance. Due to resonance, the pi-electron charge in these compounds gets delocalised. This delocalisation of electrons results in the stronger bonds than the normal pi- bonds and sigma bonds.
Because aromatic compounds (like benzene) are flat, they stack well, and so have higher melting and boiling points than corresponding alkanes and alkenes.
Answered by | 30 Sep, 2013, 10:22: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM