CBSE Class 10 Answered
Yeast cells obtain energy under anaerobic conditions by a process called alcoholic fermentation. In alcoholic fermentation, pyruvic acid is broken down into ethanol and carbon dioxide. Alcoholic fermentation is an anaerobic fermentation process similar to glycolysis that begins with the sugar glucose. The last enzyme of glycolysis, lactate dehydrogenase, is replaced by two enzymes in alcoholic fermentation. These two enzymes, pyruvate decarboxylase and alcoholic dehydrogenase, convert pyruvic acid or pyruvate into carbon dioxide and ethanol in alcoholic fermentation.
The correct chemical equation for alcoholic fermentation is as follows:
C6H12O6 + Zymase → 2 C2H5OH + 2 CO2
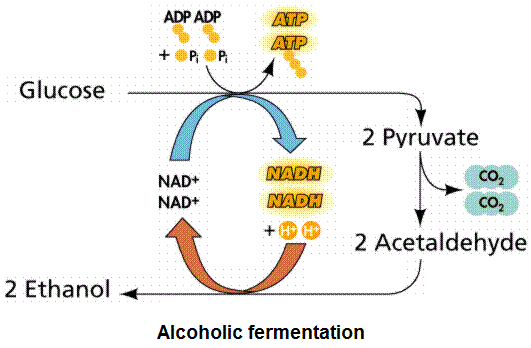
Initially, it takes ATP energy to begin the process that will only later generate a net gain in ATP. A net gain of two ATPs is obtained for each molecule of glucose degraded. The net gain of two ATPs is not realized until the tenth enzyme in the series, pyruvate kinase catalyzes phosphoenolpyruvate to ATP and pyruvic acid (pyruvate). This means that alcoholic fermentation does not have any gain in energy (ATP) until the tenth enzymatic breakdown.





