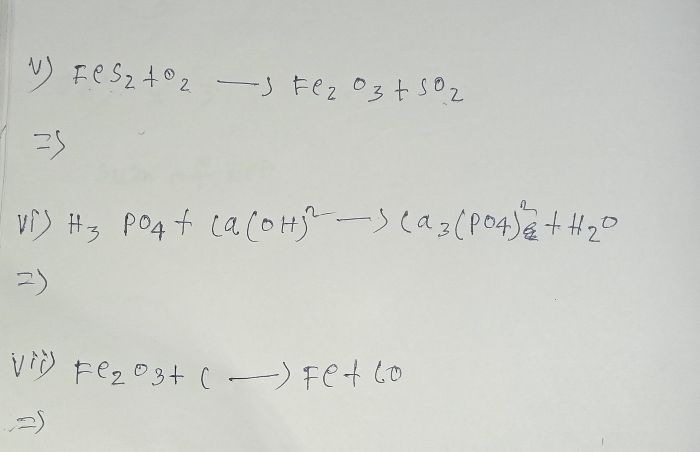CBSE Class 10 Answered
Giving chemical equation answer the following: (a) What happens when copper is heated in air? (b) What happens when the product obtained in above reaction is heated in hydrogen?
Asked by Topperlearning User | 18 Mar, 2015, 08:53: AM
(a) When copper is heated in air, it reacts with the oxygen of air to form a black compound copper oxide.
2Cu + O2 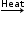 2CuO
2CuO
(b) When copper oxide formed in above reaction is heated in hydrogen, then copper oxide is reduced and brown copper metal is obtained.
CuO + H2 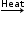 Cu + H2O
Cu + H2O
Answered by | 18 Mar, 2015, 10:53: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 11:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 11:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 06:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 05:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 04:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 02:11: PM
CBSE 10 - Chemistry
Asked by simratkaurr1 | 07 Jun, 2021, 12:55: PM