CBSE Class 12-science Answered
(i) The splitting of the degenerate d orbitals into eg and t2g orbitals due to the presence of ligands in a definite geometry is called crystal field splitting. It is denoted by ![]() .
.
(ii) Linkage isomerism arises in coordination compounds containing ambidentate ligands in which two different atoms of the same ligand can form coordinate bond with metal ion.
![]()
(iii) Ligands which can bind the central metal atom through two different atoms are called ambidentate ligands.
Example: SCN- ,NCS-
Or
![]()
![]()
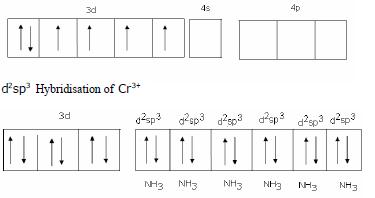
Structural shape - Octahedral
Hybrid Orbitals- d2sp3
Magnetic behaviour- Diamagnetic
![]()
![]()
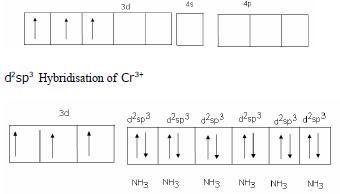
Structural shape - Octahedral
Hybrid Orbitals- d2sp3
Magnetic behavior - Paramagnetic
![]()
![]()
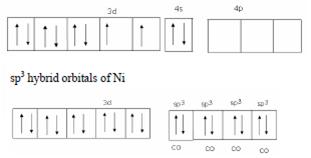
Structural shape- tetrahedral
Hybid orbitals- sp3
Magnetic behaviour- Diamagnetic



