CBSE Class 12-science Answered
Give two processes for manufacturing chlorine.
Asked by Topperlearning User | 11 Jun, 2014, 05:38: PM
(i) Deacon’s process: Chlorine can be prepared by oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of catalyst CuCl2.
4HCl +O2 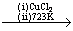 2Cl2 + 2H2O
2Cl2 + 2H2O
(ii) Electrolytic process: Chlorine is obtained by the electrolysis of concentrated NaCl solution, also called brine. In this process Chlorine is liberated at anode of the electrolytic cell.
Answered by | 11 Jun, 2014, 07:38: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by nonuhasan2 | 20 Nov, 2021, 12:05: PM
CBSE 12-science - Chemistry
Asked by ecstayatra | 07 Apr, 2020, 08:53: PM
CBSE 12-science - Chemistry
Asked by dwivedipradeep093 | 02 May, 2019, 09:21: PM
CBSE 12-science - Chemistry
Asked by prashantrairohit | 10 Feb, 2019, 09:25: AM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:49: AM
CBSE 12-science - Chemistry
Asked by Balbir | 22 Jun, 2018, 02:16: PM
CBSE 12-science - Chemistry
Asked by Balbir | 19 Jun, 2018, 06:33: PM
CBSE 12-science - Chemistry
Asked by my3shyll | 18 Apr, 2018, 06:46: PM
CBSE 12-science - Chemistry
Asked by scholarreema | 14 Mar, 2018, 12:22: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM






