CBSE Class 12-science Answered
Give me answer
Asked by yash01298 | 26 Jul, 2019, 07:49: PM
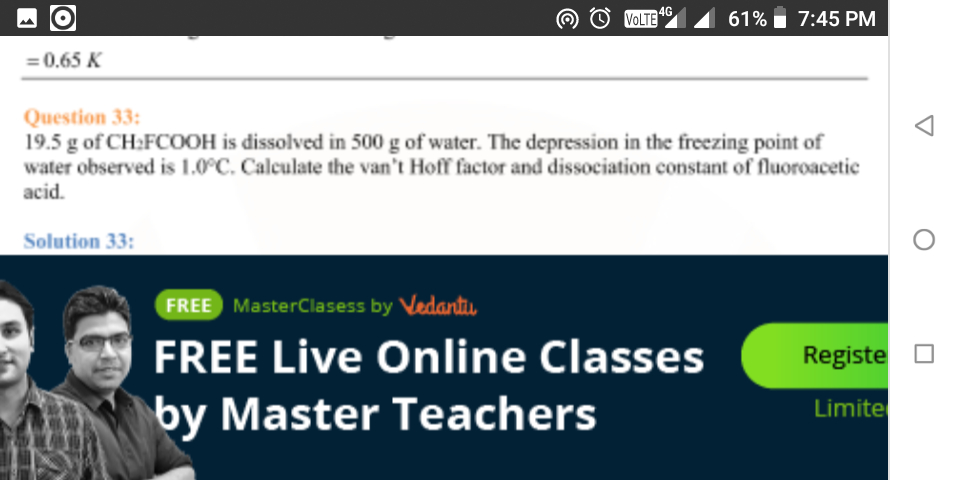
Given:
Wsolute =19.5 gm
Wsolvent = 500 gm
ΔTf = 1 ºC
Kf = 1.86 K kg/mol
Molecular weight can be calculated by using formula;
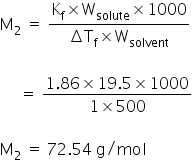
Calculated molecular weight of CH2FCOOH is,
12+(1×2) + 19 +12+(16×2)+1 = 78 g/mol
The van't Hoff factor is ,
i = 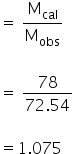
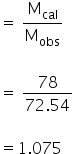
To calculate dissociation constant,
The equation is,
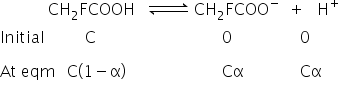
Total moles = C -Cα +Cα+Cα
=C(1+α)
We know,
van't Hoff factor, i = 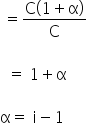
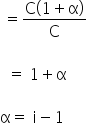
We have, i = 1.075
So, α = 1.075 - 1
= 0.075
Degree of dissociation = 0.075
Concentration, C is calculated as,
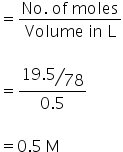
Dissociation constant, Ka is
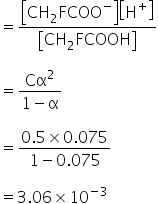
van't Hoff factor is 1.075
Dissociation constant is 3.06×10-3
Answered by Varsha | 29 Jul, 2019, 11:35: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 07:36: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by navadeepnavadeep242 | 19 Mar, 2024, 08:56: PM
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by hanihope27 | 01 Mar, 2024, 08:33: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM












