ICSE Class 10 Answered
Give appropriate scientific reasons :
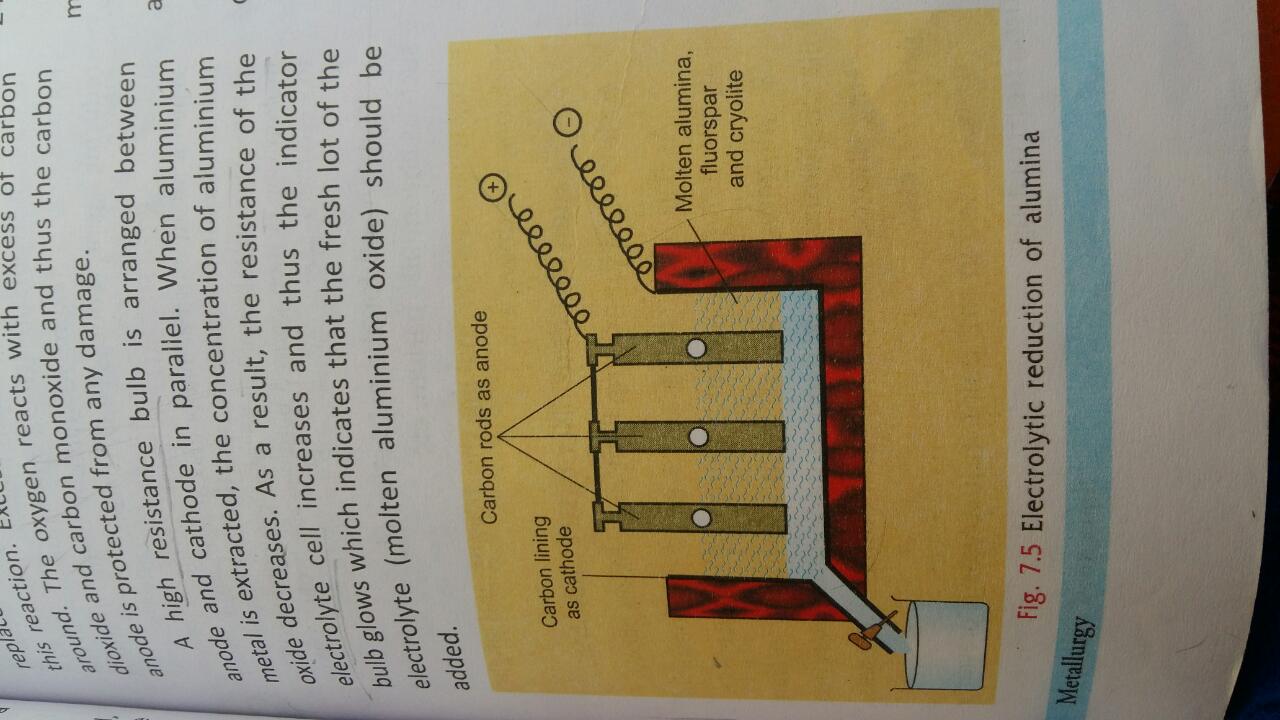
Zinc oxide can be reduced to zinc by using carbon monoxide , but aluminium oxide cannot be reduced by a reducing agent .
Asked by rickykumar00001 | 23 Feb, 2016, 07:16: PM
Metals of low reactivity cannot displace high reactive metals from their salts. Also, it has affinity for oxygen. For this reason oxides of aluminium cannot be reduced easily either by carbon, hydrogen or carbon monoxide.
Zinc oxide can be reduced to zinc by using carbon monoxide because oxides of zinc is placed below aluminium and can be reduced on heating with carbon, carbon monoxide and hydrogen. Zinc is mostly available in its carbonates and sulphides. So this metal carbonates and metal sulphides initially get converted into oxide form. Hence Zinc oxide reduced using common reducing agents like carbon, carbon monoxide and hydrogen.
Answered by Hanisha Vyas | 24 Feb, 2016, 11:26: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jamsandekar.leena | 17 Mar, 2019, 07:12: PM
ICSE 10 - Chemistry
Asked by Tech guides by | 29 Jan, 2019, 06:35: PM
ICSE 10 - Chemistry
Asked by ruthikreddy2004 | 06 Dec, 2018, 03:55: PM
ICSE 10 - Chemistry
Asked by 21janhvi.verma | 24 Sep, 2018, 12:32: PM
ICSE 10 - Chemistry
Asked by Prvati | 26 Jan, 2018, 07:18: AM
ICSE 10 - Chemistry
Asked by lovemaan5500 | 06 Jan, 2018, 03:11: PM