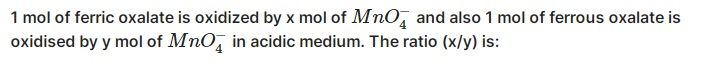CBSE Class 11-science Answered
give answer

Asked by harshilthakkarhhhhhh | 21 May, 2020, 04:37: PM
Option (C) is correct.
Given:
Volume of H2 = 12 L
= 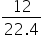
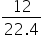
= 0.54 mol
Volume of Cl2 = 11.2 L
= 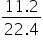
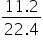
= 0.5 mol
The reaction is
H2 + Cl2 → 2HCl
1 1 2
1 mole of H2 combines with 1 mol of Cl2 to form 2 mol of HCl
Cl2 is the limiting reagent.
so 0.5 mol of Cl2 will give 1 mol of HCl
1 mol of HCl is 22.4 L of HCl
So, H2 left = 12- 11.2 = 0.8 L
HCl formed will be = 22.4 L
Answered by Varsha | 22 May, 2020, 06:09: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM