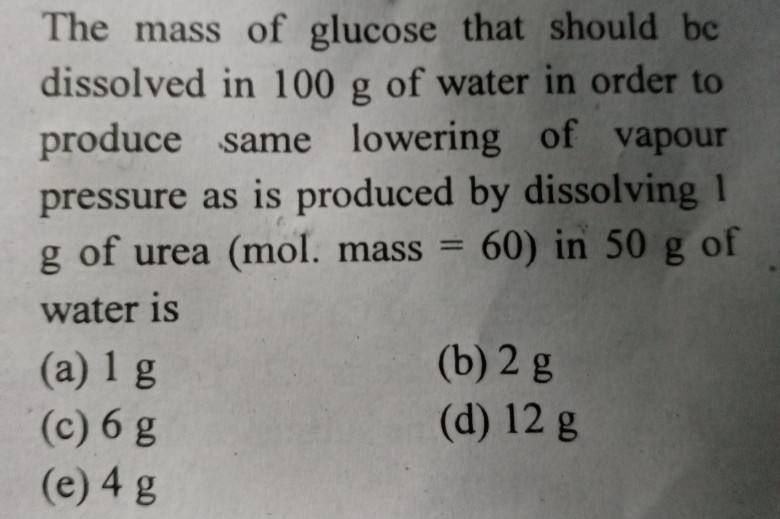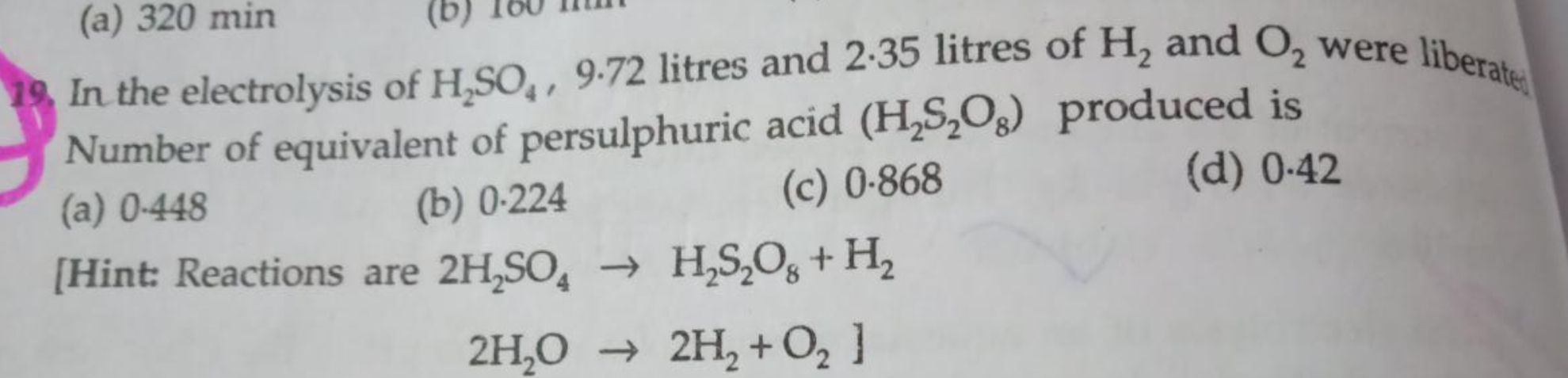NEET Class neet Answered
 G (Gibb's free energy) for every solution is said to be less than 0. but isn't it false to say that as in the non ideal solution with positive deviation the gibbs free energy should increase?
G (Gibb's free energy) for every solution is said to be less than 0. but isn't it false to say that as in the non ideal solution with positive deviation the gibbs free energy should increase?
Asked by vasu | 17 Mar, 2020, 07:07: AM
Ideal solutions are made by spontaneous process so in spontaneous process-
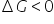
In Non ideal solution, Sponatnity decreases so 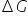 increases.
increases.
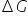 increases.
increases.
Answered by Ravi | 19 Mar, 2020, 08:05: PM
NEET neet - Chemistry
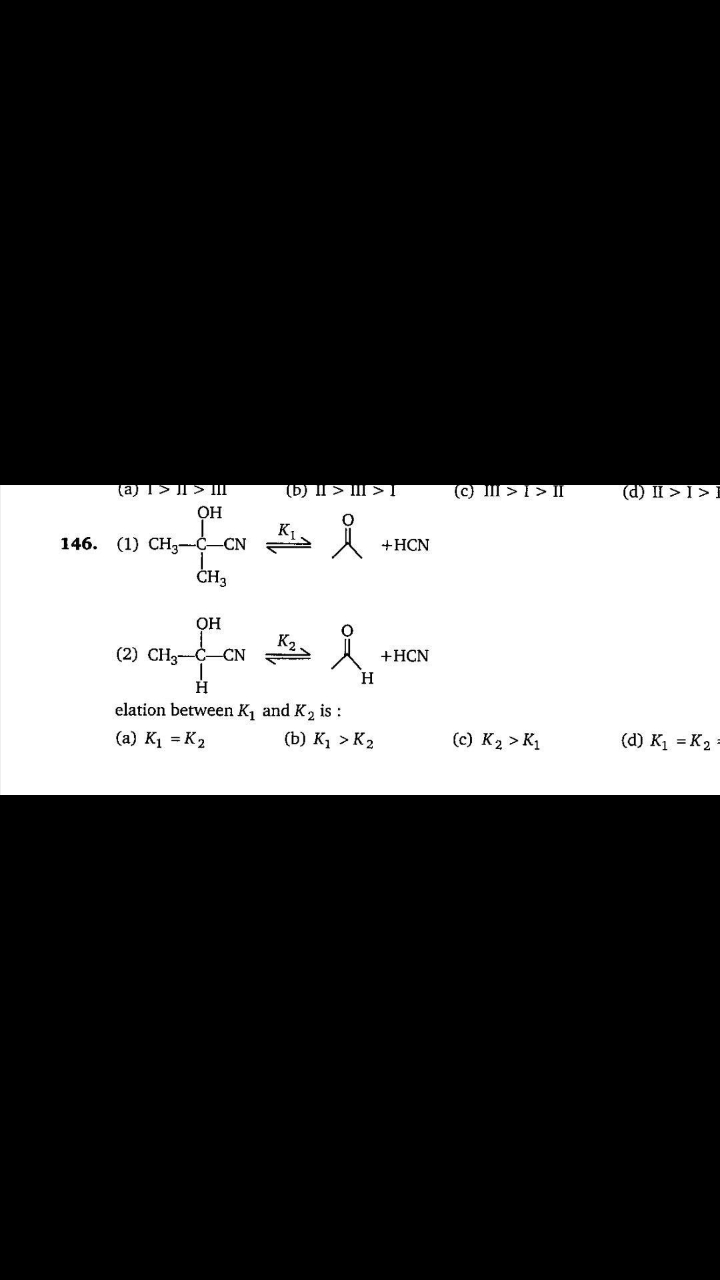
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by prakriti12oct | 28 Apr, 2020, 01:31: AM