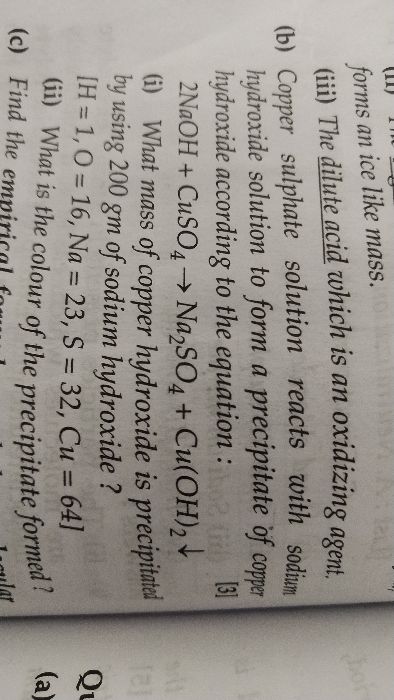ICSE Class 10 Answered
FIND THE NUMBER OF HYDROGEN ATOMS IN 0.25 MOLES OF H2SO4 (HYDROCHLORIC ACID). PLEASE ANSWER THIS QUESTION SOON. PLEASE.
Asked by kandappan | 08 Sep, 2018, 10:41: AM
As we know, 1 mole of H2So4 has 2 moles of hydrogen atoms, 1 mole of sulphur atom and 4 moles of the oxygen atoms.
For 2 moles,
1 : 2 = 0.25 : x
therefore x = 0.50 moles
Now, one mole contains 6.02 X 1023 atoms
hence,
1 : 6.02 X 1023 = 0.5 : x
x = 3.011 X 1023 hydrogen atoms.
Answered by Ramandeep | 10 Sep, 2018, 12:30: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM