CBSE Class 12-science Answered
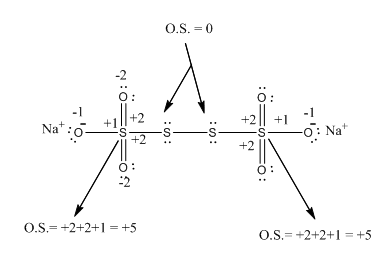
Find the difference in the oxidation numbernumbers present of the two types of sulphur present in Na2S4O6 [sodium - 2 sulphur - 4 oxygen - 6]. (Hint :- sulphur-sulphur linkages present)
Asked by acv27joy | 08 Oct, 2018, 09:08: AM
In the case of Na2S4O6, if we observe the structure of this compound it is found that there are two types of sulphur atoms
1) O.S. of first sulphur: sulphur is attached to the same sulphur atoms hence there is no change in the electronic arrangement of this sulphur atom, therefore, its O.S. is zero.
2) O.S. of second sulphur: This atom of sulphur is attached to the three oxygen atoms two are doubly bonded and one is singly bonded
Therefore for doubly bonded oxygen will exert the charge +2 and for singly bonded oxygen will +1. hence on total, the O.S. of Second sulphur is +5.
Answered by Ramandeep | 08 Oct, 2018, 11:51: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by prathyushagn1 | 09 Dec, 2020, 08:12: AM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 31 Aug, 2020, 08:24: PM
CBSE 12-science - Chemistry
Asked by sha.bijoy17 | 07 Aug, 2020, 11:55: AM
CBSE 12-science - Chemistry
Asked by Shambhuhd79 | 22 Jun, 2020, 11:09: AM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Feb, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by smit230503 | 04 Feb, 2020, 08:56: PM
CBSE 12-science - Chemistry
Asked by monishadubey202 | 08 Jan, 2020, 03:42: PM
CBSE 12-science - Chemistry
Asked by Chakshu29saini | 17 Sep, 2019, 06:19: PM
CBSE 12-science - Chemistry
Asked by bjayanta | 24 Mar, 2019, 08:56: PM
CBSE 12-science - Chemistry
Asked by himanshuneb | 28 Jan, 2019, 10:33: PM