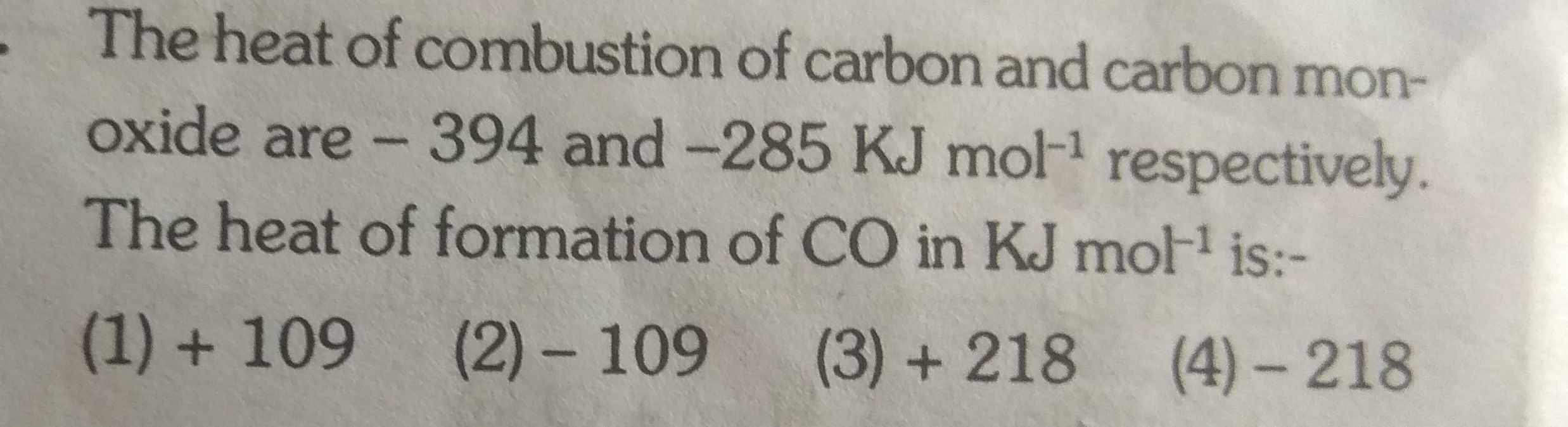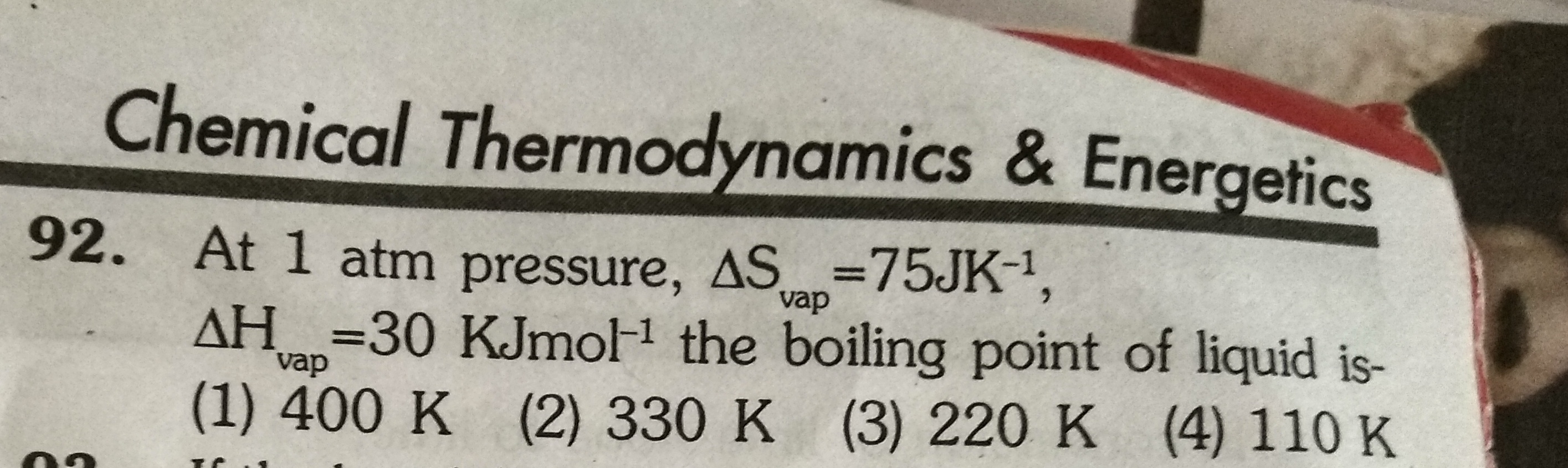JEE Class main Answered
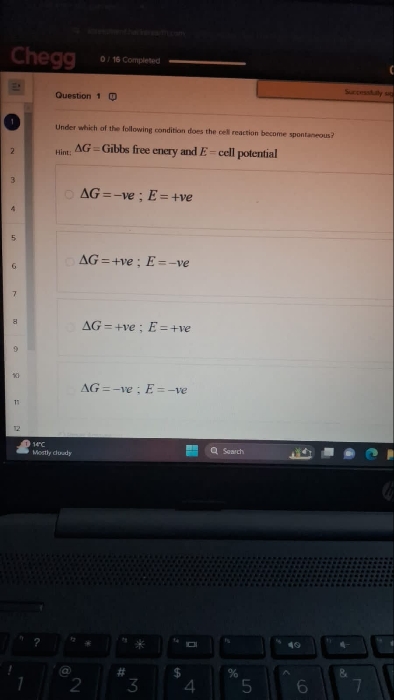
Find internal energy

Asked by ashutosharnold1998 | 03 Nov, 2019, 08:22: PM
Given:
For the change:
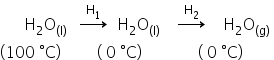
ΔH = H1+ H2
n = 1 mole
T = 100 °C
H1 = nCdT
= 1× (-4.2)×18×100
= -7560 J/mol
= -7.56 kJ/mol
H2 = -6 kJ/mol
ΔH = -7.56 + (-6)
ΔH = -13.56 kJ/mol
In the process the volume change is negligible
Therefore, ΔH = ΔU +PΔV
ΔV =0
Change in internal energy = -13.56 kJ/mol
Answered by Varsha | 05 Nov, 2019, 11:47: AM
JEE main - Chemistry
Asked by ashutosharnold1998 | 03 Nov, 2019, 08:22: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 31 Oct, 2019, 07:16: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 10 Aug, 2019, 12:10: AM
JEE main - Chemistry
Asked by Ranjeetgupta26068 | 19 May, 2019, 10:14: PM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 05:23: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 05:48: PM