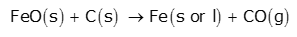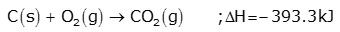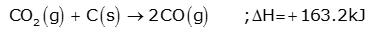CBSE Class 12-science Answered
- Extraction of iron from its oxides:
- Theory of reduction process:
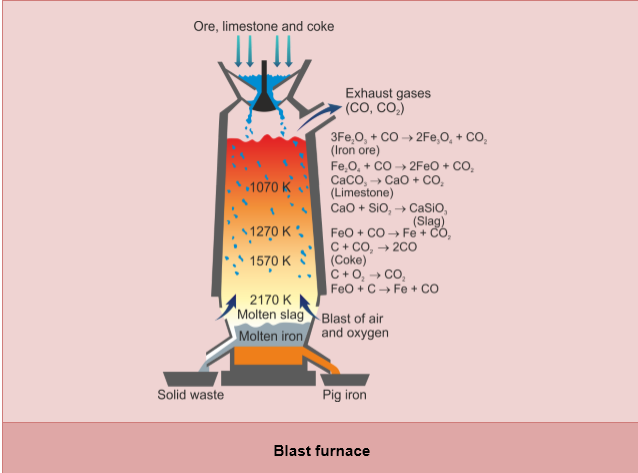
- Oxide ores of iron, after concentration through calcination/roasting, are mixed with limestone and coke and fed into a Blast furnace from its top.
- The oxide is reduced to the metal. The reduction takes place as,
- Reactions taking place in the furnace:
(a) Zone of combustion:
Near the tuyeres, small pipes through which a blast of hot air is introduced, coke burns to form carbon dioxide.
Since the reaction is exothermic, a lot of heat is produced and the temperature is around 2170K.
(b) Zone of heat absorption:
This is the lower part of the furnace and temperature is between 1423–1673K. The CO2 formed, meets the descending charge. The coke present in the charge reduces CO2 to CO.
This reaction is endothermic; therefore, the temperature gradually falls to 1423K.
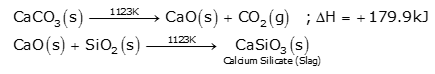
(c) Zone of slag formation:
It is the middle part of the furnace. The temperature is around 1123K. In this region, limestone decomposes to form CaO and CO2. This CaO combines with silica to form fusible calcium silicate slag.
(d) Zone of reduction:
This is the upper part of the furnace. The temperature is between 500-900K. Here, ores are reduced to Fe by CO.
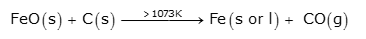
In the middle part of the furnace, the temperature is 900-1500K. At the temperature above 1073K, FeO reduces to Fe by carbon.
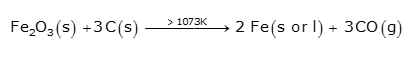
Finally, the direct reduction of iron ore takes place completely to iron by carbon above 1073K.
(e) Zone of fusion:
- This is the lower part of the furnace.
- The temperature here is between 1423-1673K.In this region, spongy iron with impurities and CaSiO3 slag melts.
- Both molten iron and molten slag form two separate layers.
- The molten slag is lighter hence forms the upper layer while molten iron is heavier hence forms the lower layer.
- The iron obtained from this furnace contains 4% carbon and many impurities in a small amount. This is called pig iron.