CBSE Class 12-science Answered
Explain, with
the help of a nuclear reaction in each of the following cases, how the
neutron to proton ratio changes during (i) alpha- decay (ii) beta-decay?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
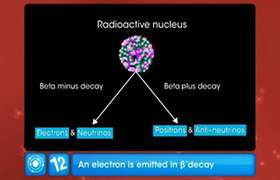
(i)
![]()
Neutron to proton ratio before ![]() - decay =
- decay = ![]()
Neutron to proton ratio after ![]() - decay =
- decay = ![]()
As ![]()
Thus, the neutron to proton ratio increases in an ![]() -decay.
-decay.
(ii)
![]()
Neutron to proton ratio before ![]() -decay =
-decay = ![]()
Neutron to proton ratio after ![]() -decay =
-decay = ![]()
As ![]()
Thus the neutron to proton ration decreases in a ![]() -decay
-decay
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Physics
Asked by arjunsah797 | 16 May, 2022, 02:17: PM
CBSE 12-science - Physics
Asked by merinlijo_20002 | 17 Jun, 2020, 10:05: AM
CBSE 12-science - Physics
Asked by rajubarman | 01 Dec, 2019, 10:03: AM
CBSE 12-science - Physics
Asked by alanpeter9611 | 23 Feb, 2019, 07:47: PM
CBSE 12-science - Physics
Asked by alanpeter9611 | 22 Feb, 2019, 11:24: PM
CBSE 12-science - Physics
Asked by sd2021667 | 04 Dec, 2018, 04:46: PM
CBSE 12-science - Physics
Asked by silladech | 16 Nov, 2018, 11:14: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 09 Jul, 2014, 04:06: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 09 Jul, 2014, 04:15: PM






 , find the number of nuclei present after 8 days?
, find the number of nuclei present after 8 days?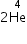 →
→  + ..............
+ ..............
