CBSE Class 12-science Answered
Explain why does a galvanic cell stop working over a period of time?
Asked by Topperlearning User | 22 Jun, 2016, 02:36: PM
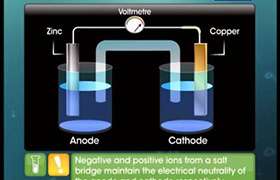
In a galvanic cell, the transfer of electrons from anode to cathode leads to a net positive charge around the anode and a net negative charge around the cathode. The positive charge around anode prevents electrons to flow out from it so the potential difference becomes zero and the cell stops after sometime.
Answered by | 22 Jun, 2016, 04:36: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM
CBSE 12-science - Chemistry
Asked by Poojanisha1988 | 19 Jul, 2023, 09:59: PM
CBSE 12-science - Chemistry
Asked by jajimuji2306 | 03 Apr, 2022, 01:38: PM
CBSE 12-science - Chemistry
Asked by Harshfarwaha | 23 Jul, 2020, 03:27: PM
CBSE 12-science - Chemistry
Asked by sourabhkumar9923 | 19 May, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by ssharondaniel | 27 Jul, 2019, 06:22: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 28 Feb, 2019, 06:57: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 12 Sep, 2018, 05:40: PM