CBSE Class 12-science Answered
The first experimental proof of the wave nature of electron was demonstrated in 1927 by two American physicists C.J Davison and L.H Germer. The basis of their experiment was that since the wavelength of an electron is of the order of spacing of atoms of a crystal, a beam of electrons shoes diffraction effects when incident on a crystal.

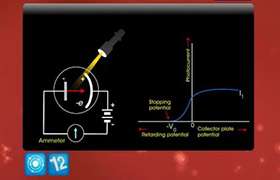
Experimental arrangement
The figure shows the experimental setup.
Electrons are emitted by a hot filament 'F' by thermionic emission. They are passed between the anode and the cathode, which accelerates the electrons. These accelerated electrons were made to fall on a nickel crystal normally. The beam of electrons is diffracted by the crystal and received at an angle f by a detector D. the intensity of the diffracted electrons is measured by the detector as a function of angle q and also the scattered electron current.
The study of this is represented in the graph.
As voltages increases first attained a peak and decreases there after. The existence of peak in the graph can be explained as due to constructive interference of waves scattered from atoms in different planes of the crystal. This looks something similar to diffraction pattern of X-rays. Hence, Davison and Germer's experiment stands as an evidence of the wave nature of electrons.
The peak value in the graph can be explained by Bragg's formula
2dSinq = hl
where h is the order of diffraction,
d is the atomic spacing between successive crystal planes and 'q' is the angle at which strong reflection takes place.
Hope this clarifies your doubt.
Regards
Team
Topperlearning