CBSE Class 10 Answered
Explain Co valent Bond
Asked by akshay.beloshe | 05 Jul, 2018, 12:31: PM
Covalent Bond:
The chemical bond formed due to mutual sharing of electrons between the given pairs of atoms of non-metallic elements.
Conditions for Formation of Covalent Compounds
- Both atoms should have high electronegativity, electron affinity and ionisation energy.
- The electronegative difference between the two combining atoms should be negligible.
- Both atoms should have four or more electrons in their outermost shell.
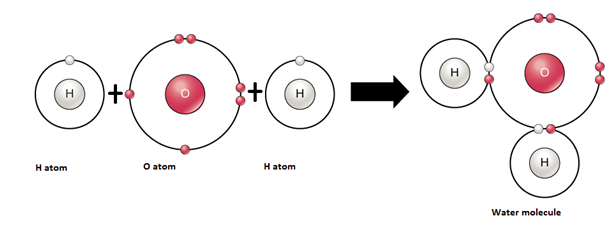
4. Formation of Water – Covalent Compound
|
Atom |
Electronic configuration |
Nearest noble gas |
To attain the stable electronic configuration of the nearest noble gas |
|
Hydrogen |
11H [1] |
Helium [2] |
Hydrogen needs one electron to complete the duplet. |
|
Oxygen |
168O [2,6] |
Neon [2,8] |
Oxygen needs two electrons to complete the octet. |
Answered by Ramandeep | 05 Jul, 2018, 12:47: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM
CBSE 10 - Chemistry
Asked by sunitha4503 | 28 Jul, 2020, 10:25: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 09:41: PM