Even though the distance between C-C single bond is more (154pm) and between double is less (133pm), the pi bond in C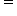 C can be broken more easily. Why is it so? Shouldn't it be difficult to break?
C can be broken more easily. Why is it so? Shouldn't it be difficult to break?
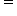 C can be broken more easily. Why is it so? Shouldn't it be difficult to break?
C can be broken more easily. Why is it so? Shouldn't it be difficult to break?Pi bonds are easier to break than sigma bonds because of two reasons.
- In the formation of σ bond, the end-on overlap of orbitals is more efficient than the side-on overlap of orbitals to form a pi bond.
- The electrons involved in a sigma bond are between the two nuclei. They are as closer to the nuclei and hence the nuclei have a strong grip on the electrons, therefore it is difficult to break a sigma bond.The electrons in a pi bond are further away from the nuclei. The nuclei experience a weaker attraction to the electrons; hence it is easier to break a pi bond.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
