CBSE Class 12-science Answered
Estimate the mass of copper metal produced during the passage of 5A current through CuSO4 solution for 100 minutes.the molar mass of Cu is 63.5g mol-1.
Asked by jaiswalishika960 | 04 Jun, 2019, 02:49: PM
Given:
I = 5 A
t = 100 min
= 100× 60
= 6000 sec
The reaction is,
Cu2+ + 2e− → Cu
We know,
Q = It
= 5 × 6000
= 3 × 104 C
As 2×96500 C requires to deposite 63.5 gm of Cu.
So, 3 × 104 C will give,
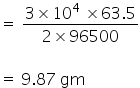
Mass of copper metal deposited is 9.87 gm
Answered by Varsha | 04 Jun, 2019, 04:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM
CBSE 12-science - Chemistry
Asked by sy985326 | 21 Feb, 2024, 04:23: AM
CBSE 12-science - Chemistry
Asked by rishitadekaraja123 | 07 Feb, 2024, 08:41: AM
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by skmdsajid04 | 14 Jan, 2024, 09:23: AM
CBSE 12-science - Chemistry
Asked by samskruthikrishn | 12 Jan, 2024, 10:11: AM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by 2507king2006 | 03 Oct, 2023, 07:12: AM
CBSE 12-science - Chemistry
Asked by keerthana.d.cst.2022 | 22 Aug, 2023, 08:18: PM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM







