CBSE Class 11-science Answered
Enthalpy of combustion of carbon graphite and carbon diamond is -293.5(kJ/mol) and - 670(kJ/mol) respectively. Calculate conversion of graphite into diamond.
Asked by Topperlearning User | 14 Aug, 2014, 09:58: AM
Aim equation: C (graphite) → C (diamond)
Given;
Graphite C (graphite) + O2(g) → CO2(g)  = -290.5 (kJ/mol) ----1
= -290.5 (kJ/mol) ----1
 = -290.5 (kJ/mol) ----1
= -290.5 (kJ/mol) ----1Diamond C (diamond) + O2(g) → CO2(g)  = -670.5 (kJ/mol) ----2
= -670.5 (kJ/mol) ----2
 = -670.5 (kJ/mol) ----2
= -670.5 (kJ/mol) ----2Subtract 2 from 1
C (graphite) → C (diamond) ‚ąÜH = + 380(kJ/mol)
Answered by | 14 Aug, 2014, 11:58: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by paulnaveed202 | 17 Dec, 2020, 12:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 04:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:58: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:15: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:41: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM



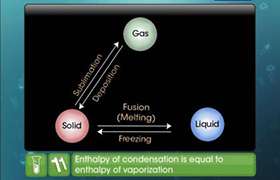
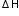 from: Enthalpy changes of formation and bond enthalpies.
from: Enthalpy changes of formation and bond enthalpies.