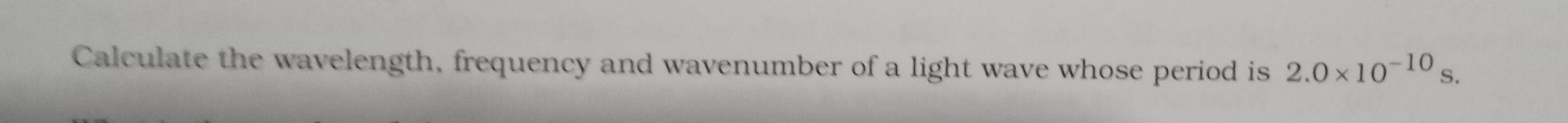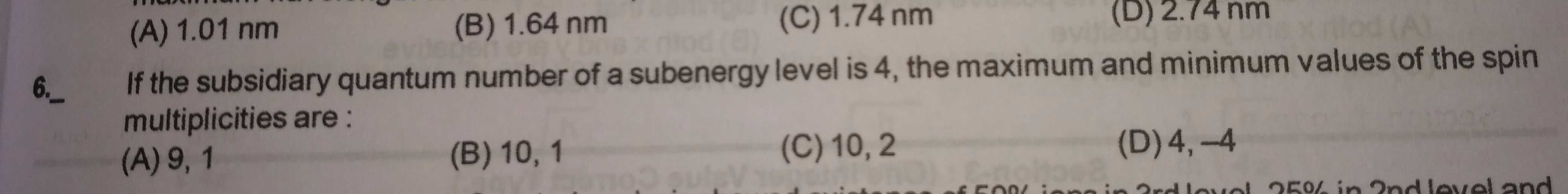JEE Class main Answered
Electronic configuration of copper
Asked by architasinha8434 | 20 May, 2019, 03:26: PM
Atomic number of Copper (Cu) is 29.
Thus electronic configuration will be,
1s2 2s2 2p6 3s2 3p6 4s1 3d10 ... (by Aufbau's principle)
Here, as the d orbital requires one electron to get complete, d orbital pulls the electron from s orbital. Hence there is one electron in 4s orbital and 10 electrons in 3d orbital.
Answered by Shiwani Sawant | 20 May, 2019, 04:21: PM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 11:27: PM
JEE main - Chemistry
Asked by pratap62437 | 19 Feb, 2024, 12:48: PM
JEE main - Chemistry
Asked by sayushman087 | 01 Feb, 2024, 10:28: AM
JEE main - Chemistry
Asked by marthalamanoharreddy65 | 17 Dec, 2023, 10:26: AM
JEE main - Chemistry
Asked by aftab01561 | 02 Jul, 2022, 11:35: PM
JEE main - Chemistry
Asked by katakamsettygayathri | 11 Jun, 2022, 09:49: PM
JEE main - Chemistry
Asked by vkanirudh2 | 30 May, 2022, 09:58: PM
JEE main - Chemistry
Asked by Joydeep | 14 Jul, 2019, 12:51: PM
JEE main - Chemistry
Asked by mahadev2471 | 08 Jun, 2019, 11:51: AM
JEE main - Chemistry
Asked by architasinha8434 | 20 May, 2019, 03:26: PM