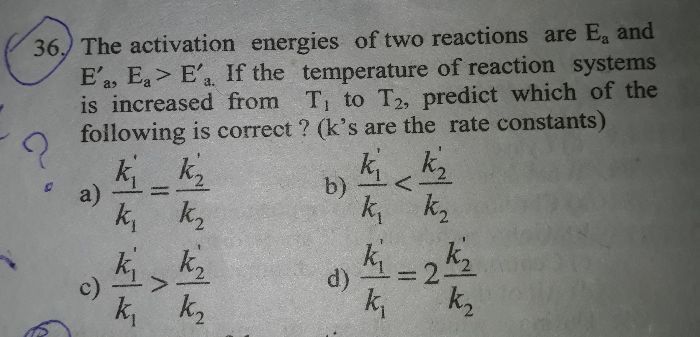CBSE Class 12-science Answered
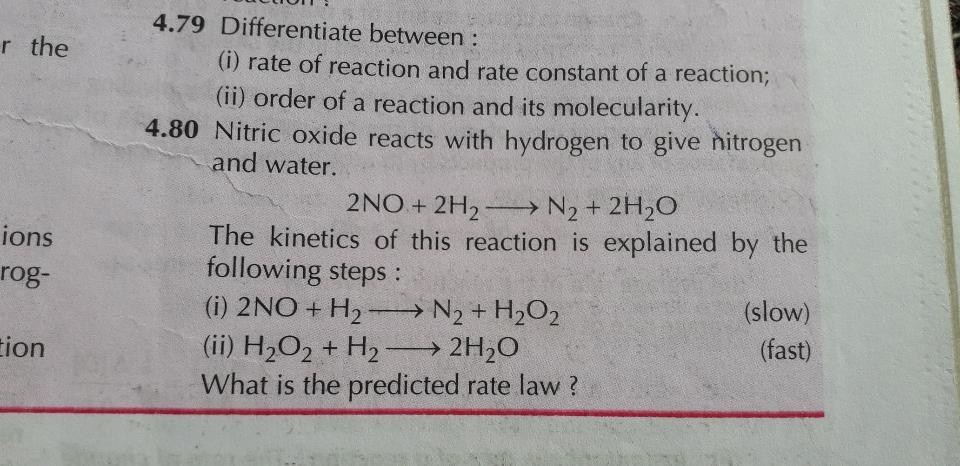
Determine the rate law and the value of k for the following reaction using the data provided.
2n2o5=4no2+o2
[n2o5] initial rate
0.093 4.84 x 10^-4
0.084 4.37 X 10^-4
0.224 1.16 x 10^-3
Asked by lekhakarthikeyan | 26 Aug, 2018, 08:00: PM
Given:
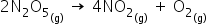
[N2O5] Initial rate
0.093 4.84 x 10-4 --------- (1)
0.184 9.37 X 10-4 ---------(2)
0.224 1.16 x 10-3 ---------(3)
From equation (1) and (2), it is observed that when the concentration of [N2O5] doubles the rate is also doubled.
Also, from equation (1) and (3) it is observed that when the concentration of [N2O5] triple the rate is also tripled.
Therefore,
Rate =k[N2O5]
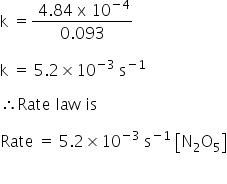
k = 5.2×10-3 s-1
Rate law:
Rate =5.2×10-3 s-1 [N2O5]
Answered by Varsha | 27 Aug, 2018, 04:38: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM
CBSE 12-science - Chemistry
Asked by amritha2960 | 13 May, 2020, 08:26: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 02 May, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by leelakrishnapallapotu143 | 29 Mar, 2020, 07:27: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 30 Jan, 2020, 03:29: PM