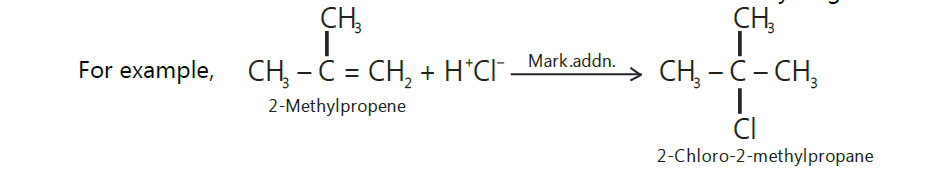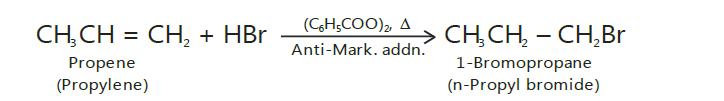describe Markovikov rule and kharash effect
Markovnikov’s rule.
Markovnikov, a Russian chemist, studied a large number of such addition reactions and postulated an empirical rule in 1869 which is known after him as Markovnikov’s rule.
The rule states that,
“The addition of unsymmetrical reagents such as HX, H2O, HOX, etc. to unsymmetrical alkenes
occurs in such a way that the negative part of the addendum (i.e., adding molecule) goes to that
carbon atom of the double bond which carries lesser number of hydrogen atoms.”
Kharasch effect
It should be noted that Markovnikov’s rule is not always followed.
In the presence of peroxides such as benzoyl peroxide, the addition of HBr (but not ofHCl or HI) to unsymmetrical alkenes takes place contrary to Markovnikov’s rule.
This is known as Peroxide effect or Kharasch effect.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Classification of Elements and Periodicity in Properties
- 2 Chemical Bonding and Molecular Structure
- 3 States of Matter
- 4 Equilibrium
- 5 Hydrogen
- 6 Hydrocarbons
- 7 Environmental Chemistry
- 8 Solutions
- 9 Chemical Kinetics
- 10 Surface Chemistry
- 11 Biomolecules
- 12 Polymers
- 13 Chemistry in Everyday Life
- 14 Atomic Structure
- 15 Chemical Thermodynamics
- 16 Redox Reactions and Electrochemistry
- 17 p-Block Elements
- 18 d - and f - Block Elements
- 19 Some Basic Principles of Organic Chemistry
- 20 Organic Compounds Containing Halogens
- 21 Organic Compounds Containing Oxygen
- 22 Organic Compounds Containing Nitrogen
- 23 Co-ordination Compounds
- 24 Purification and Characterisation of Organic Compounds
- 25 s-Block Element (Alkali and Alkaline Earth Metals)
- 26 Solid State
- 27 Some Basic Concepts in Chemistry
- 28 General Principles and Processes of Isolation of Metals
- 29 Principles Related to Practical Chemistry