CBSE Class 11-science Answered
The gas equation is an equation used in chemical equations for calculating the changes in the volume of gases when pressure and temperature both undergo a change,
thereby giving a simultaneous effect of changes of temperature and pressure on the volume of a given mass of dry gas.
According to Boyle's law:
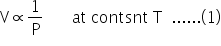
According to Charles' law:

According to Avogadro's law:

where n is the number of molecules
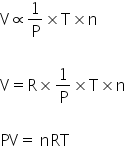
where, R is molar gas constant.
If the volume of a given mass of a gas changes from V1 to V2, its pressure changes from P1 to P2 and its temperature changes from T1 to T2, then
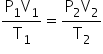
The above equation is called the gas equation. This equation is also called the combined gas equation.