CBSE Class 11-science Answered
Define lattice energy?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Lattice energy is the measure of the forces of attraction between the ions. It is defined as the amount of energy required to break one mole of a crystal into its free ions.
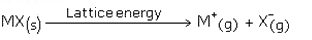
The larger the forces of attraction, the greater will be lattice energy. The lattice energy also depends upon the size of the ion and its charge. For the cation of same valency, ionic solids having the same anion will display a decrease in the lattice energy with increase in size of the cation. This is due to decrease in forces of attraction between them.
The larger the forces of attraction, the greater will be lattice energy. The lattice energy also depends upon the size of the ion and its charge. For the cation of same valency, ionic solids having the same anion will display a decrease in the lattice energy with increase in size of the cation. This is due to decrease in forces of attraction between them.
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreyasharma94.11dga | 23 Mar, 2022, 11:42: PM
CBSE 11-science - Chemistry
Asked by mrassam2711 | 15 Jan, 2021, 11:54: PM
CBSE 11-science - Chemistry
Asked by sg908580 | 21 Jan, 2020, 09:16: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:37: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:37: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 01:43: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




