CBSE Class 10 Answered
Dear sir/madame,
Can you please explain what sp hybridized mean?
Thank you
Asked by 123cmb123 | 28 Nov, 2018, 10:40: PM
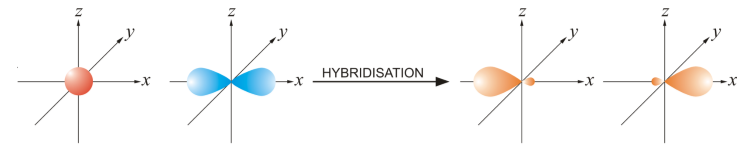
sp Hybridisation
- Intermixing of one 's' and one 'p' orbital of almost equal energy to give two identical and degenerate hybrid orbitals is called 'sp' hybridisation.
- These sp-hybrid orbitals are arranged linearly by making a 180° angle.
- They possess 50% 's' and 50% 'p' character.
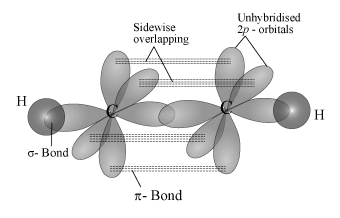
Structure of C2H2:
- The ground state configuration of ‘C’ being 1s2 2s2 2px1 2py1 has only two unassociated electrons. Carbon makes four bonds because its valency is four.
- For this, 4 unpaired electrons are required. Hence, it promotes its 2s electron to the empty 2pz orbital in the excited state.
- So, the excited state electronic configuration of carbon is 1s2 2s1 2px1 2py1 2pz1. Each carbon atom undergoes ‘sp’ hybridisation by using 2s and 2p orbitals in the excited state to give two half-filled ‘sp’ orbitals, which are arranged linearly.
Answered by Ramandeep | 29 Nov, 2018, 10:23: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM
CBSE 10 - Chemistry
Asked by sunitha4503 | 28 Jul, 2020, 10:25: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 09:41: PM









