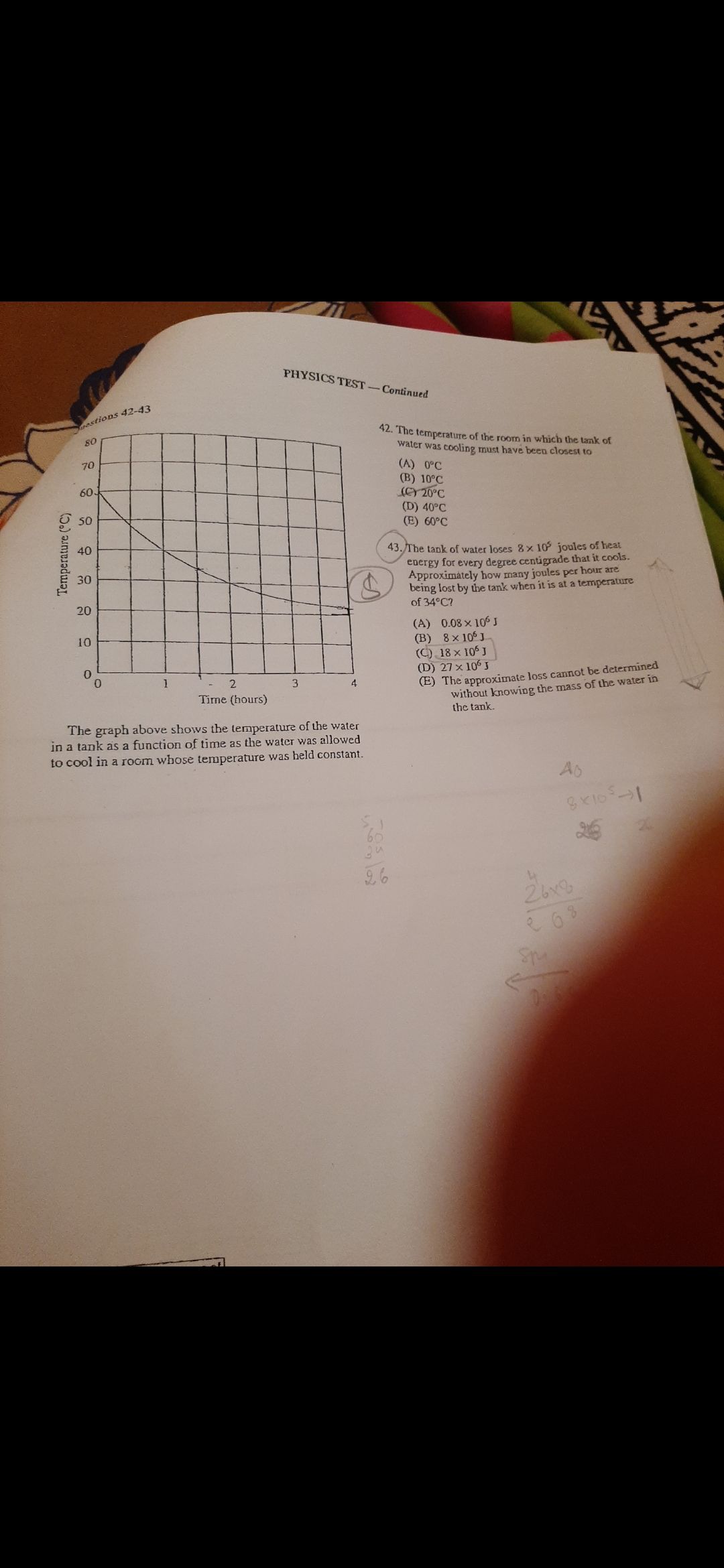
CBSE Class 12-science Answered
de broglie
Asked by | 13 Mar, 2010, 01:52: PM
(c)  (3/8)
(3/8)
de Broglie wavelength, λ = h/ (2πmkT)
(2πmkT)
where h - Planck's constant, m - mass of atom, k - Boltzmann constant, T - temperature in K.
Hydrogen molecules, H2, will have 2 protons,
Helium molecules will have 2 protons and 2 neutrons. Masses of protons and neutrons are approximately same. Hence Helium gas molecule will be TWICE massive than hydrogen molecule.
λHydrogen/λHelium =  [(mT)Hydrogen/(mT)Helium] =
[(mT)Hydrogen/(mT)Helium] =  (300/(2x400)) =
(300/(2x400)) =  (3/8)
(3/8)
Regards,
Team,
TopperLearning.
Answered by | 13 Mar, 2010, 02:48: PM
Concept Videos
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by mazhartahsildar143 | 07 May, 2020, 01:44: PM
CBSE 12-science - Physics
Asked by Hemanthrakshitha95 | 03 Mar, 2020, 02:55: PM
CBSE 12-science - Physics
Asked by shivakumarshreyas24 | 01 Mar, 2020, 08:12: AM
CBSE 12-science - Physics
Asked by khushimassey437 | 31 May, 2019, 08:41: AM
CBSE 12-science - Physics
Asked by jain.pradeep | 26 May, 2019, 06:42: PM
CBSE 12-science - Physics
Asked by manasvijha | 19 Mar, 2019, 07:17: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 08 Jan, 2019, 04:33: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:18: AM