CBSE Class 12-science Answered
Classify solids and give basis of their classification.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
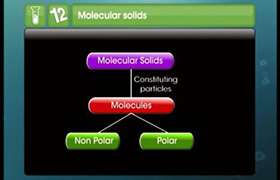
Solids are classified as crystalline and amorphous. Crystalline solids have ions, atom or molecules arranged in an orderly array and the elementary particles have a fixed site/place. The fundamental difference between single crystal and amorphous solids is the length scale over which the atoms are related to one another by translational symmetry ('periodicity' or 'long-range order'). Crystals have infinite periodicity and amorphous solids (and liquids) have no long-range order.
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by sardaarjii2012 | 31 Oct, 2021, 09:05: PM
CBSE 12-science - Chemistry
Asked by karennavarpriya | 20 Aug, 2021, 01:53: PM
CBSE 12-science - Chemistry
Asked by apoorvaks26 | 10 May, 2021, 09:24: AM
CBSE 12-science - Chemistry
Asked by someshmule5 | 05 Jan, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 16 Oct, 2020, 01:18: PM
CBSE 12-science - Chemistry
Asked by hemanthkumarbk2356 | 02 Aug, 2020, 12:16: PM
CBSE 12-science - Chemistry
Asked by harishshrivastava855 | 21 Jun, 2020, 09:26: PM
CBSE 12-science - Chemistry
Asked by manjurana04933 | 16 Apr, 2020, 11:00: AM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 06:47: PM
CBSE 12-science - Chemistry
Asked by yaag8432 | 05 Mar, 2019, 07:28: PM