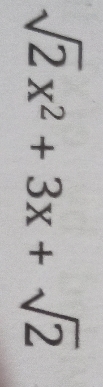CBSE Class 9 Questions and Answers
CBSE 9 - Maths
Asked by monika.vns14 | 18 Apr, 2024, 05:15: PM
CBSE 9 - History
Asked by nirajbhai228 | 16 Apr, 2024, 05:35: PM
CBSE 9 - Maths
Asked by gulshanelectronics2017 | 15 Apr, 2024, 05:35: PM
CBSE 9 - Maths
Asked by tripura78839 | 12 Apr, 2024, 09:05: PM
CBSE 9 - Maths
Asked by singhanjali0199 | 12 Apr, 2024, 08:29: PM
CBSE 9 - Geography
Asked by dhulljannat7 | 11 Apr, 2024, 06:30: PM
CBSE 9 - Civics
Asked by asmanazma2008 | 09 Apr, 2024, 03:01: PM
CBSE 9 - Civics
Asked by yanuj8700 | 07 Apr, 2024, 08:44: PM