CBSE Class 11-science Answered
Sign conventions for work:
-w – work done by system (expansion for pV work)
+w – work done on the system (compression for pV work)
Sign conventions for heat:
-q – heat transferred away from body (heat lost)
+q – heat transferred into body (heat gained)
Sign conventions for enthalpy:
ΔH = +ve – heat is absorbed – Endothermic
ΔH = -ve – heat is evolved (given out) – Exothermic
Sign conventions for entropy:
∆Suniv = ∆Ssys + ∆Ssurr;
When both ∆Ssys and ∆Ssurr are positive, ∆Suniv must be positive (so process is spontaneous). ∆Suniv is always negative (so process is nonspontaneous) when both ∆Ssys and ∆Ssurr are negative. When the signs of ∆Ssys are opposite of each other [(∆Ssys (+), ∆Ssurr (−) or vice versa], the process may or may not be spontaneous.
Sign conventions for Gibb’s free energy:
When ΔG is negative, the process or chemical reaction proceeds spontaneously in the forward direction.
When ΔG is positive, the process proceeds spontaneously in reverse.
When ΔG is zero, the process is already in equilibrium, with no net change taking place over time.
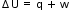 .
.