CBSE Class 12-science Answered
Can Magnesium Choride be amorphous?
Asked by Rajeev | 22 Jan, 2016, 08:09: PM
No Magnesium chloride is an ionic compound formed between magnesium metal and non-metal chlorine.
Ionic compounds are crystalline with strong electrostatic forces of attraction between oppositely charged ions.
Covalent compounds are amorphous.
Answered by Vaibhav Chavan | 25 Jan, 2016, 12:30: AM
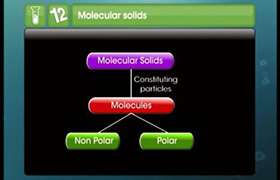
Concept Videos
CBSE 12-science - Chemistry
Asked by sardaarjii2012 | 31 Oct, 2021, 09:05: PM
CBSE 12-science - Chemistry
Asked by karennavarpriya | 20 Aug, 2021, 01:53: PM
CBSE 12-science - Chemistry
Asked by apoorvaks26 | 10 May, 2021, 09:24: AM
CBSE 12-science - Chemistry
Asked by someshmule5 | 05 Jan, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 16 Oct, 2020, 01:18: PM
CBSE 12-science - Chemistry
Asked by hemanthkumarbk2356 | 02 Aug, 2020, 12:16: PM
CBSE 12-science - Chemistry
Asked by harishshrivastava855 | 21 Jun, 2020, 09:26: PM
CBSE 12-science - Chemistry
Asked by manjurana04933 | 16 Apr, 2020, 11:00: AM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 06:47: PM
CBSE 12-science - Chemistry
Asked by yaag8432 | 05 Mar, 2019, 07:28: PM