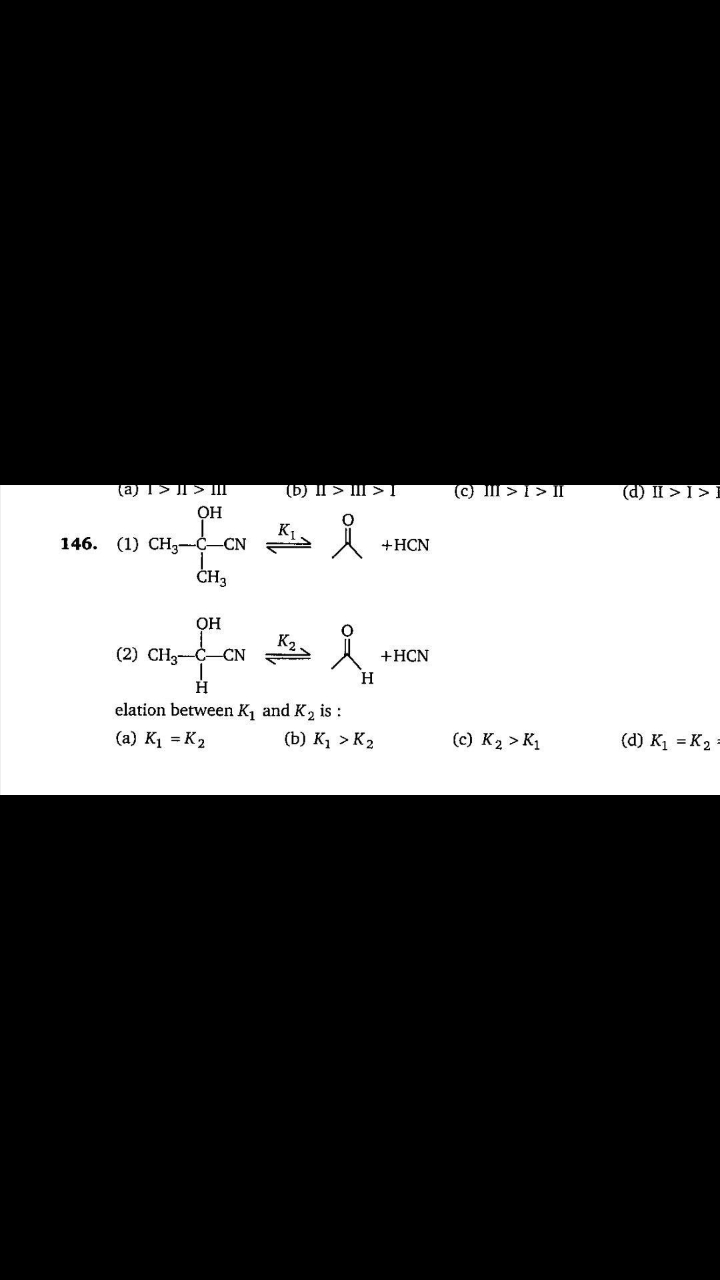
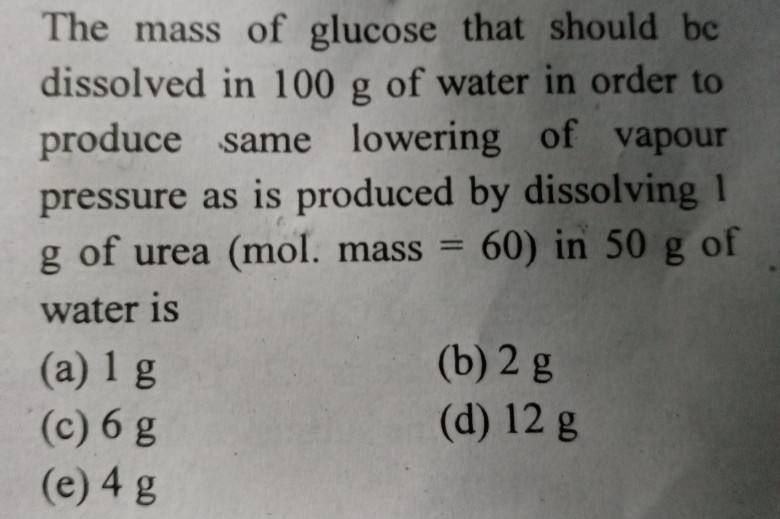NEET Class neet Answered
Calculate the normality of 250 mL aqueous solution of H2SO4 having pH -0.0
(a)0.25 N
(b)0.50 N
(c)1 N
(d)2 N
Asked by Balbir | 09 Jul, 2019, 10:25: PM
Given:
Volume of solution = 250 ml
pH of the solution = 0
We know,
pH = -log[H+]
0 =-log[H+]
Taking antilog
[H+] = 1 M
we know that,
Molaity of H2SO4 = 2Normality of H2SO4
Normality of H2SO4
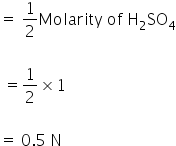
Normality of solution is 0.5 N.
Answered by Varsha | 10 Jul, 2019, 10:48: AM
Concept Videos
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 17 May, 2020, 03:07: PM