CBSE Class 12-science Answered
Calculate the density for nickel if the unit cell edge length for nickel given is 0.3524 nm and atomic mass is 58.69 g /mol. It crystallizes as a simple cube.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
The volume (V) of the unit cell is equal to the cell-edge length (a) cubed.
a =.3524nm =.352 x 10-7cm
V = a3 = (0.3524 x 10-7cm )3 =4.376 x10-23cm3
The mass of a nickel atom can be calculated from the atomic weight of
this metal and Avogadro's number. 58.69g/mol / 6.022 x 1023
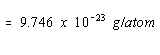
The density of nickel, =9.746 x 1023 /4.376 x 1023 = 2.23 g/cm3
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 07:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 09:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




