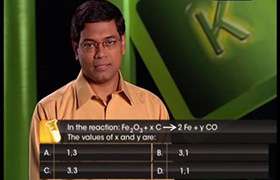CBSE Class 11-science Answered
We cannot entertain more than 5 questions in a single query. In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Step 1:
The skeleton equation is:
As2S3 + NO-3 + H+ → AsO3-4 + S + NO2 + H2O
Step 2:
Oxidation number of various atoms involved in the reaction is as follows:
3+ 2- +5 2- +5 2- 0 +4 -4
As2 S3 + N O-3 + H+ → As O3-4 + S + N O2 + H2 O
Step 3:
We can balance S by just observation. For Nitrogen oxidation number changes from +5 to +4 so it is reduced. For Arsenic (As) oxidation number changes from +3 to +5 so it is oxidised.
Step 4:
Determine the net increase in oxidation number for the element that is oxidized and the net decrease in oxidation number for the element that is reduced.
For As +3 to +5 Net change = +2
For N +5 to +4 Net change = -1
Step 5:
Determine a ratio of oxidized to reduced atoms that would yield a net increase in oxidation number equal to the net decrease in oxidation number.
As atoms would yield a net increase in oxidation number of +2 (Two electrons would be lost by one As atom.) and 2 N atom would yield a net decrease of -2. (Two N atoms would gain two electrons.)
Thus the ratio of As atoms to N atoms is 1:2.
Step 6:
To get the ratio identified in Step 5, add coefficients to the formulas which contain the elements whose oxidation number is changing.
As2S3 + 2NO-3 + H+ → AsO3-4 + S + 2NO2 + H2O
Step 7:
But still the equation is not balanced. Balance the rest of the equation by inspection.
First balance the oxygen. So the balanced equation is,
As2S3 + 10NO-3 + 4H+ → 2AsO3-4 + 3S + 10NO2 + 2H2O
Topperlearning Team.