CBSE Class 11-science Answered
Answer this question please.
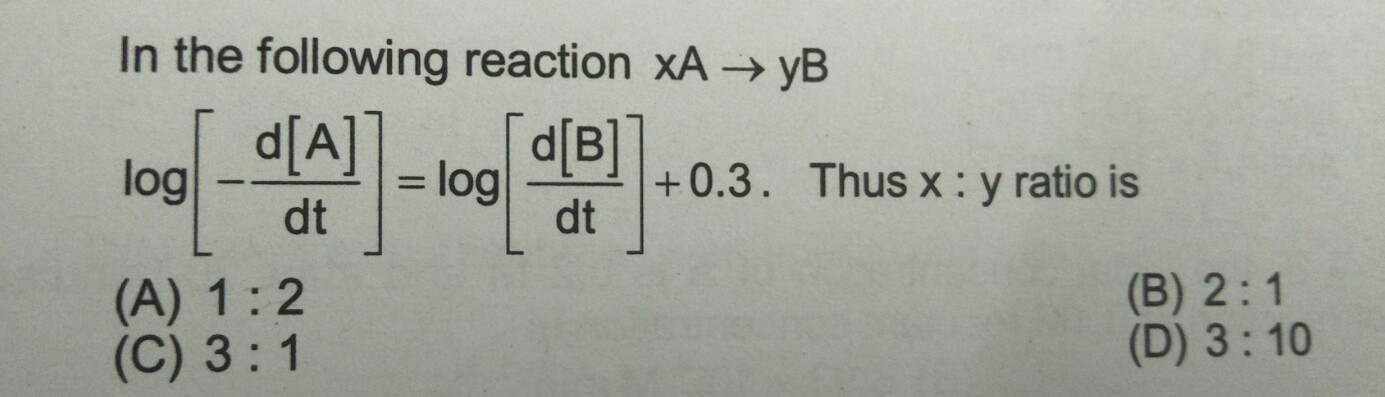
Asked by dineshchem108 | 14 Dec, 2018, 08:18: PM
The above equation can also be written as :
Log {-d[A]/dt} = Log{d[B]/dt + Log 2 (Since log 2 = 0.3010)
Removing log on both sides we get,
[A] = 2[B]
Applying rate equation to the above we get ;
-1/2 d[A]/dt = +d[B]/dt
So, x = 2 and y = 1
Ratio; x:y = 2:1
Answered by Sumit Chakrapani | 15 Dec, 2018, 10:42: AM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM













