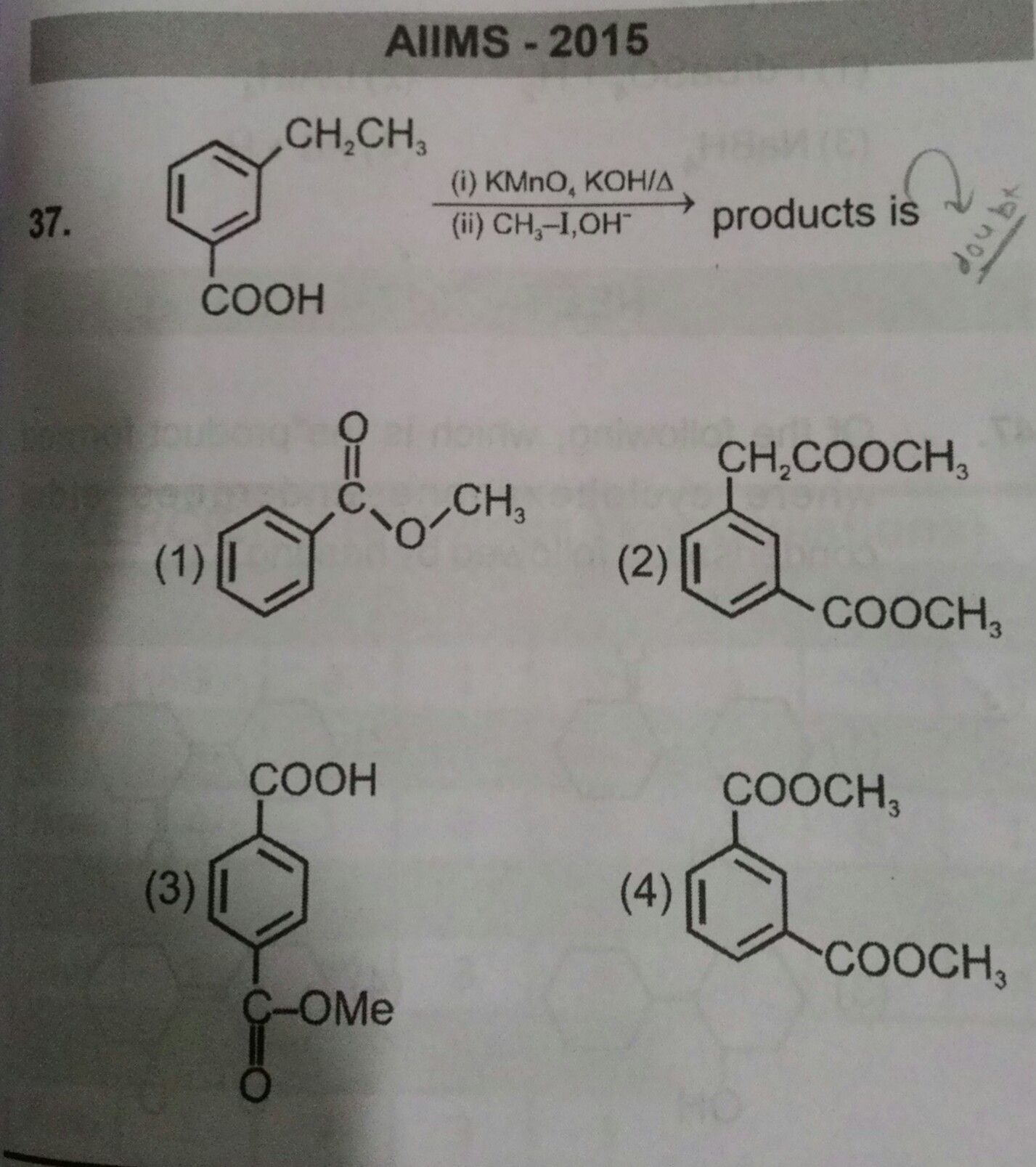
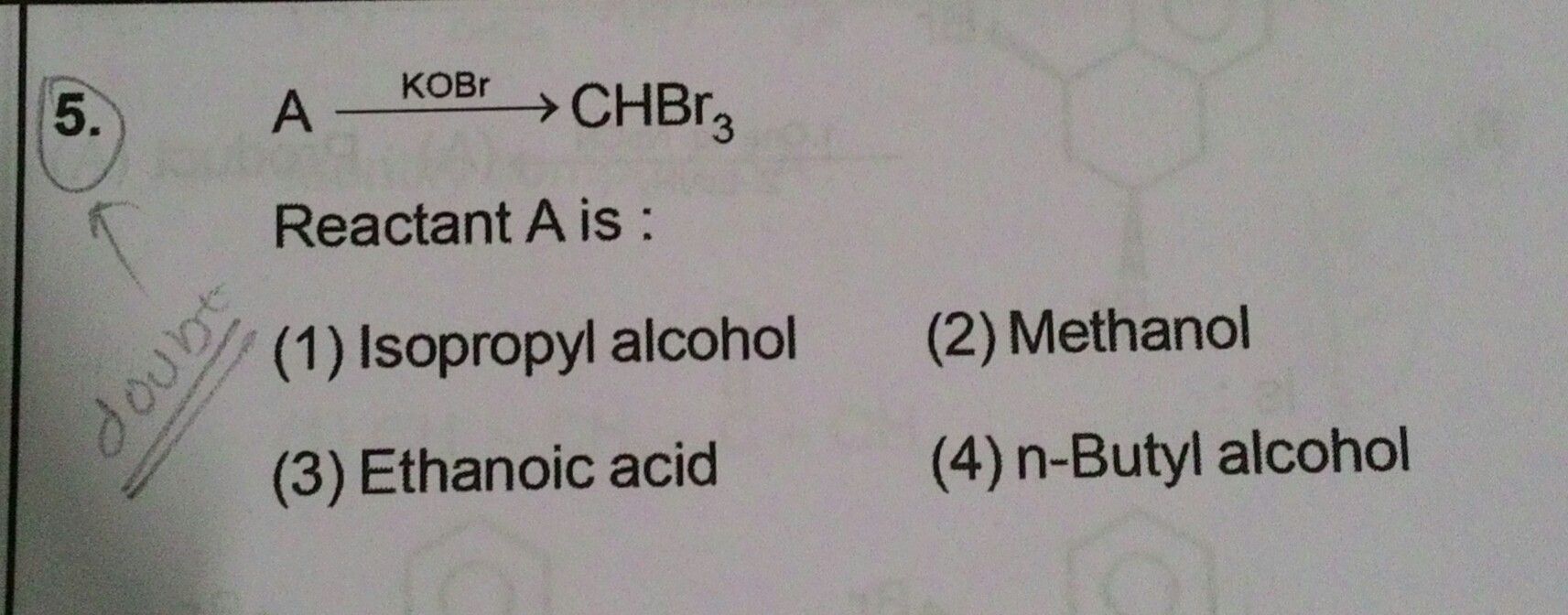NEET Class neet Answered
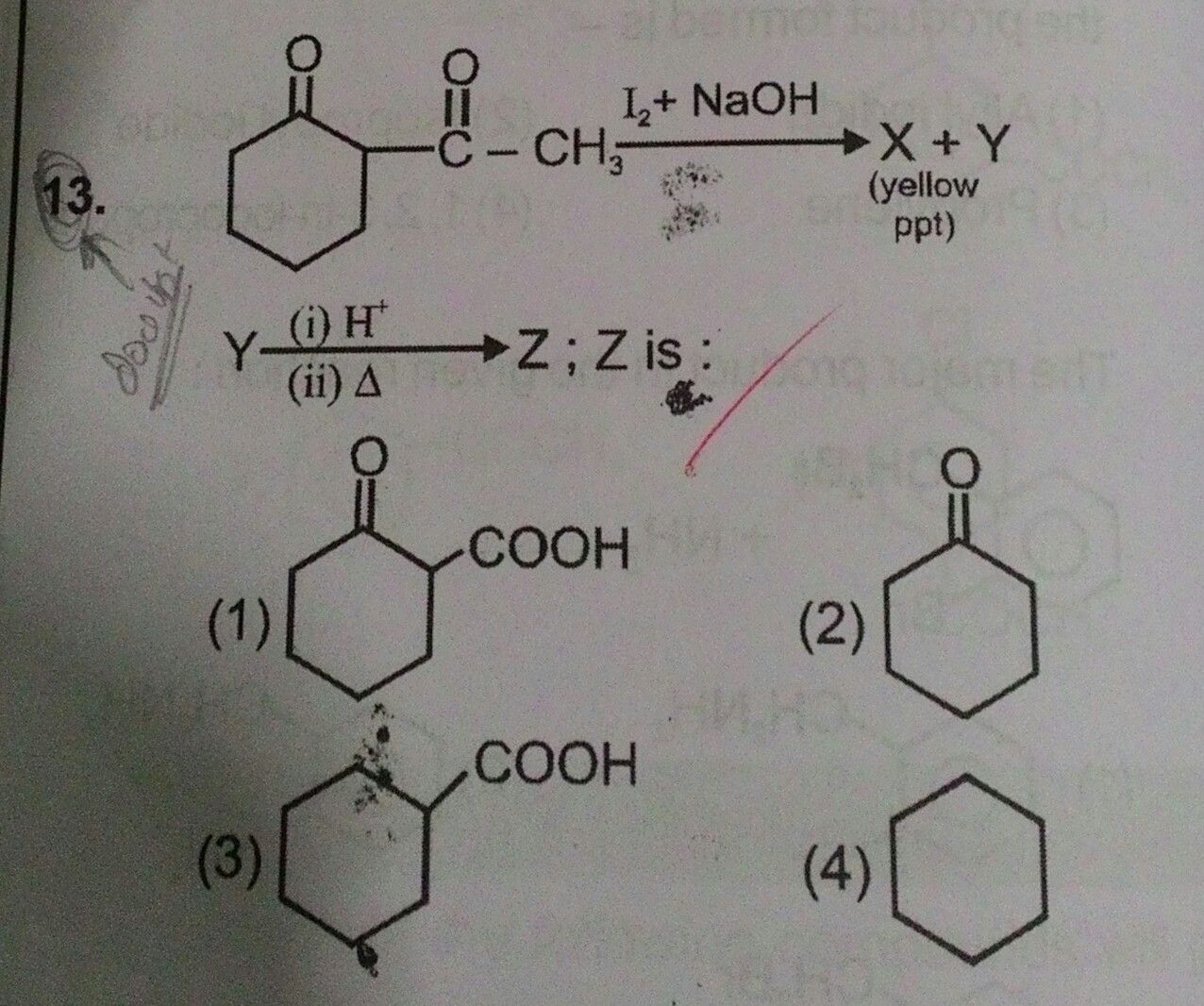
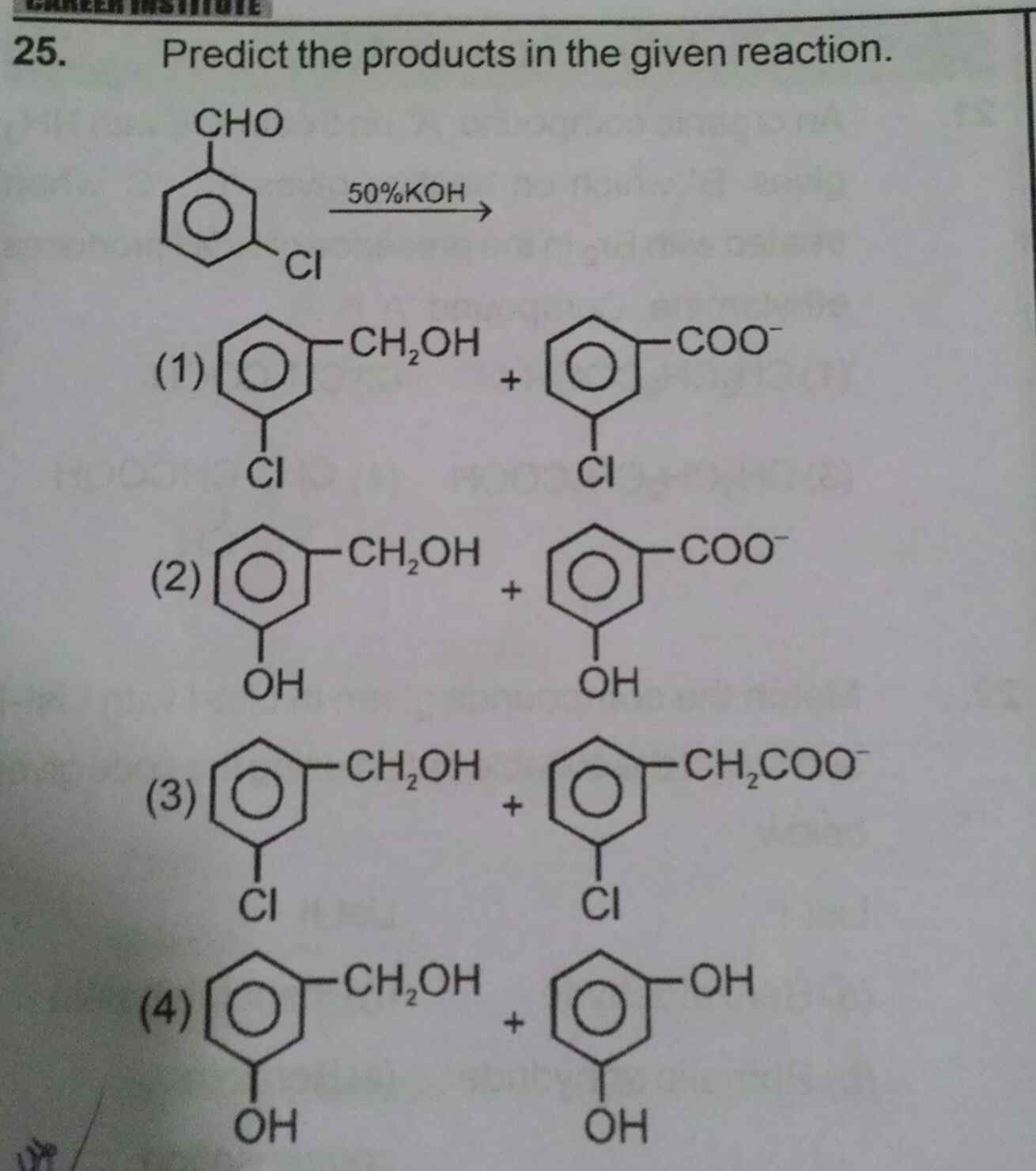
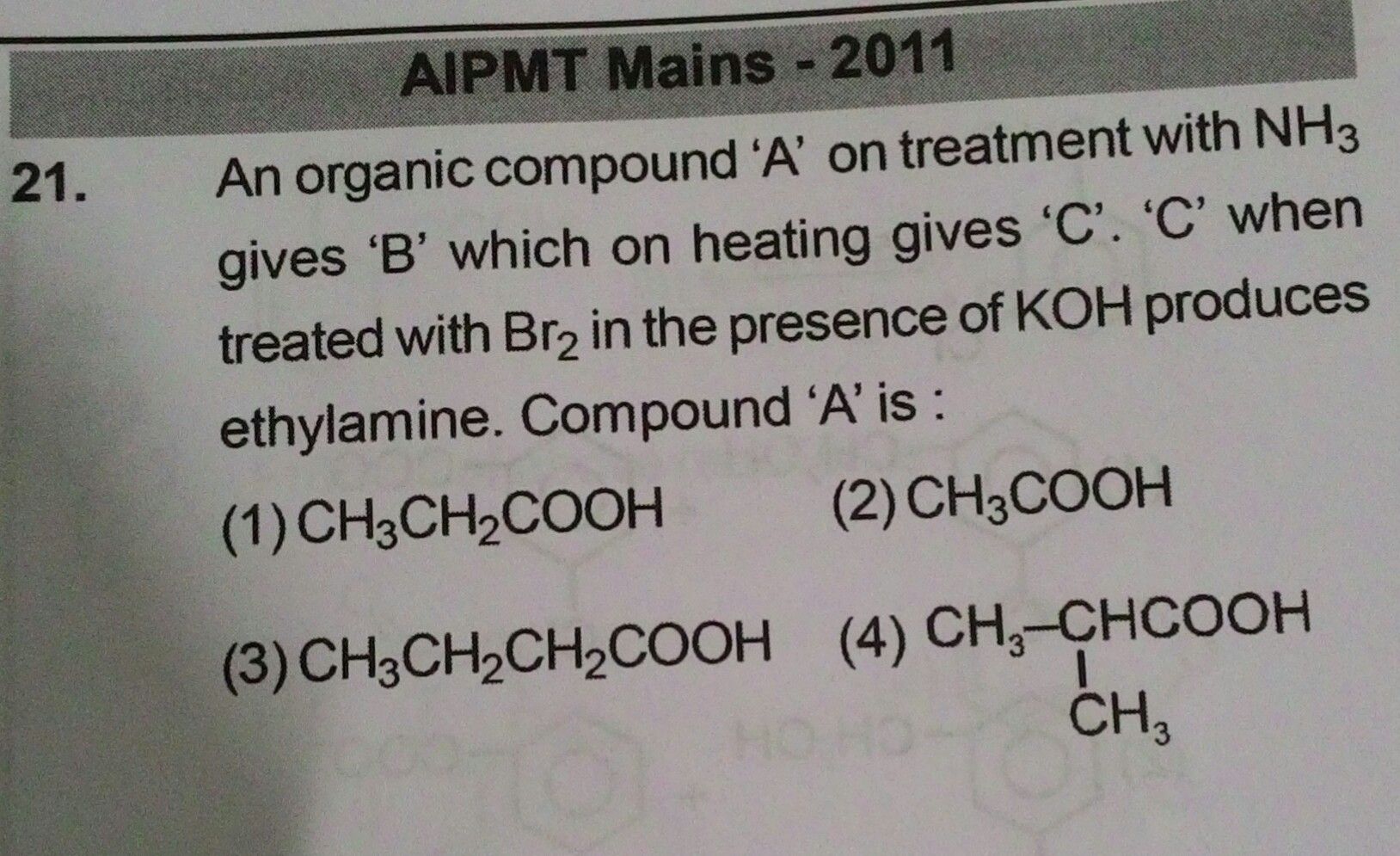
Answer is option 1 .... Plzzz explain why?【Attachment】
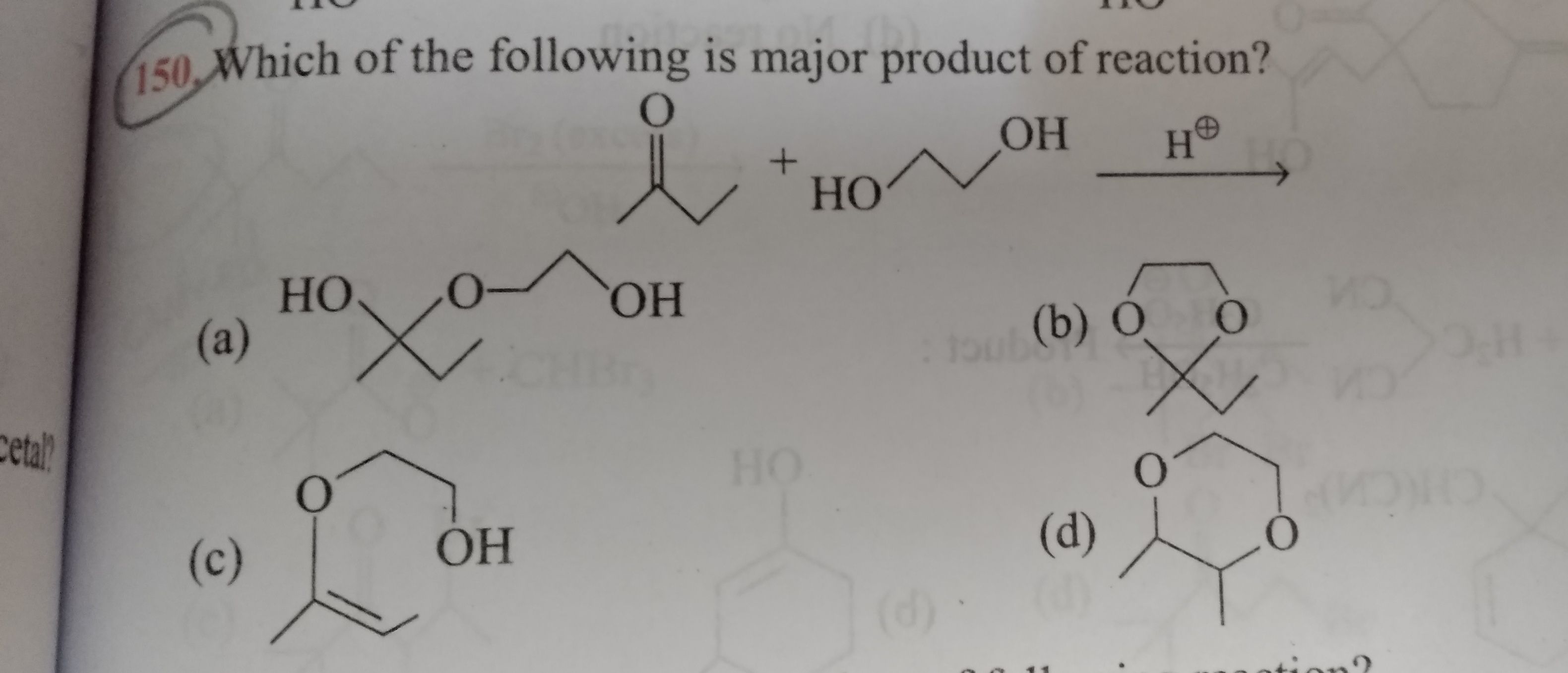
2) which of the following will form stable hydrate when reacts with H3O+
a) acetone
b) ethyl methyl ketone
c) benzaldehyde
d) 1-oxo cyclopropane
Answer is option d... Plzz explain
Asked by jhajuhi19 | 21 May, 2019, 12:35: AM
Explanation:
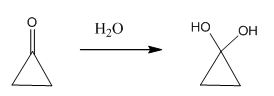
1-oxo cyclopropane will form stable hydrate because the obtained product has lesser bond angle strain than the reactant.
This is becuase generally sp2 hybridised carbon has 120º bond angle but in case of 1-oxo cyclopropane the sp2 carbon only gets 60º.
when it convertd into diols the bond angle strain is reduces to large extend.
Answered by Ramandeep | 24 May, 2019, 07:06: PM
NEET neet - Chemistry
Asked by prakriti12oct | 01 Sep, 2020, 12:09: AM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 22 Jul, 2020, 11:24: PM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 12 Jul, 2020, 10:40: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:45: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 24 Feb, 2020, 10:02: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 23 Feb, 2020, 10:26: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 20 Feb, 2020, 10:16: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 19 Feb, 2020, 10:46: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 19 Feb, 2020, 10:28: PM