CBSE Class 10 Answered
AN organic compound X on heating with conc.H2SO4 forms a compound Y which on adding of one moleclue of hydrogen in the presence of nickel forms a compound Z. one molecule of Z on combustion forms two molecules of CO2 and three molecules of H2O. identify X,Y,Z. also write their chemicl equations.
Asked by | 23 Mar, 2013, 10:08: AM
Compound X forms compound Y on reaction with H2SO4. Y is undergoing addition reaction when treated with hydrogen in the presence of Nickel. So, Y is an unsaturated compound. COmpound Z undergoing combustion and forms two molecules of CO2 and 3 molecules of H2O. This hints at ethane. The reaction is:
C2H6 + 2O2 --> 2CO2 + 3H2O
So, Z os ethane. ethane is formed on hydrogenation of ethene with one molecule of hydrogen over nickel.
C2H4 + H2 ------------> C2H6
Ethanol on dehydration with H2SO4, gives ethene
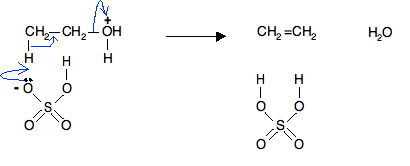
Answered by | 25 Mar, 2013, 06:10: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by agarwalkrishnam98 | 01 Oct, 2023, 08:28: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:01: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM











