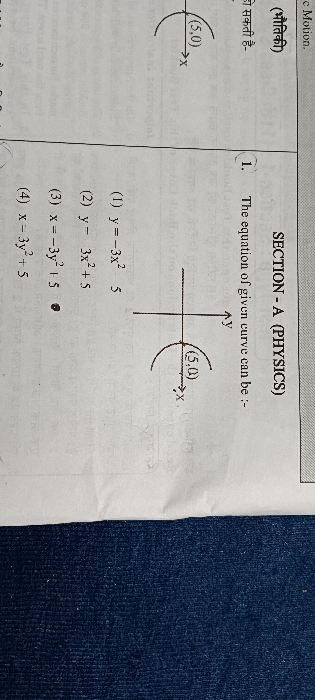NEET Class neet Answered
An ideal gas expands according to the law P^2V=constant. The internal energy of the gas-(1) Increase continuously, (2) Decrease continuously, (3) Remains constant, (4) First increase and then decrease.
Asked by patra04011965 | 19 Mar, 2019, 01:19: AM
Given,
P2V = constant
So,
P2V = k
Thus,
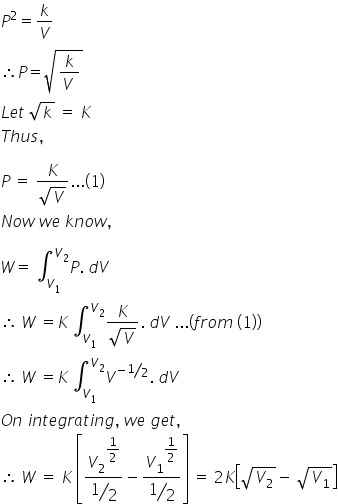
By first law of thermodynamics,
Δ U = Q + W
Q = 0
Δ U = Q + W
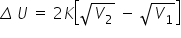
So now, as the gas is expanding,
V2 > V1
Thus, Δ U will increase continuously.
Thus, correct option is (1)
Answered by Shiwani Sawant | 19 Mar, 2019, 12:55: PM
Application Videos
NEET neet - Physics
Asked by rifabanu200715 | 02 Apr, 2024, 08:24: PM
NEET neet - Physics
Asked by labdhisangoi101 | 30 Mar, 2024, 02:51: PM
NEET neet - Physics
Asked by onemile96 | 30 Mar, 2024, 01:04: PM
NEET neet - Physics
Asked by 69sayyappan | 29 Mar, 2024, 10:44: AM
NEET neet - Physics
Asked by hindujaguttula890 | 29 Mar, 2024, 08:34: AM
NEET neet - Physics
Asked by anandibastavade555 | 26 Mar, 2024, 11:06: PM