CBSE Class 11-science Answered
An element X has the following isotopic composition..200 X:90%,199 X:8%,202 X:2%.The weighed average atomic mass of the naturally occurring element X is closest to(in amu)?
Asked by pb_ckt | 22 Apr, 2019, 11:20: PM
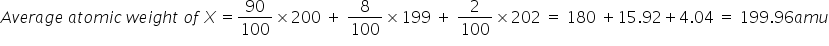
Answered by Sumit Chakrapani | 24 Apr, 2019, 01:48: AM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM













