CBSE Class 12-science Answered
Let, wavelength of an electron and a photon be λe and λp respectively.
λe = λp = 1 nm = 1 × 10–9 m
Planck's constant, h = 6.63 × 10–34 Js
(a)The momentum of an elementary particle is given by de Broglie relation
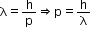
It is clear that momentum depends only on the wavelength of the particle. Since the wavelengths of an electron and a photon are equal, both have an equal momentum.
![]()
(b)The energy of a photon is given by the relation:
![]()
Where,
Speed of light, c = 3 × 108 m/s
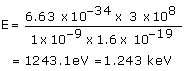
Therefore, the energy of the photon is 1.243 keV
(c) The kinetic energy (K) of an electron having momentum p, is given by the relation:
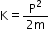
Where,
m = Mass of the electron = 9.1 ×10–31 kg
p = 6.63 × 10–25 kg m s–1
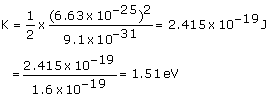
Hence, kinetic energy of the electron is 1.51 eV.




