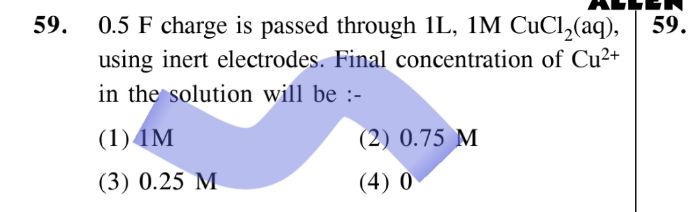
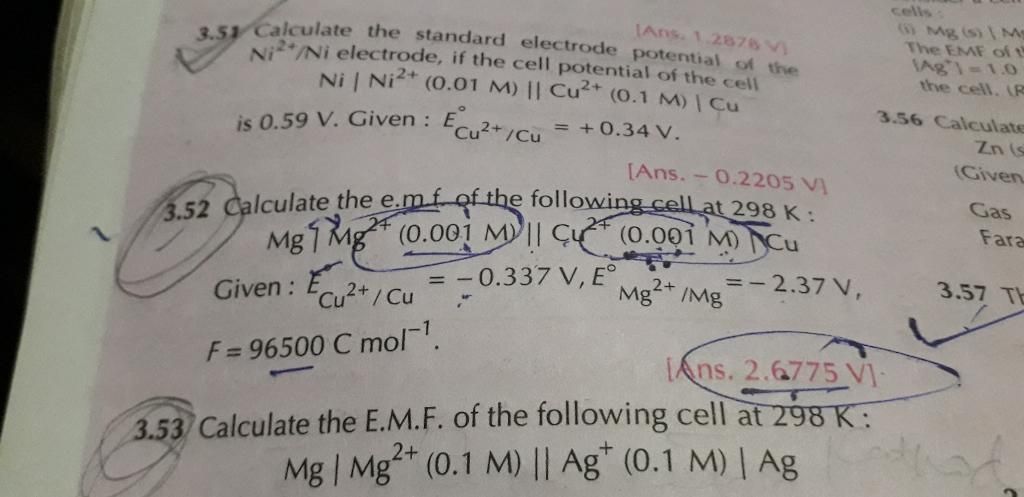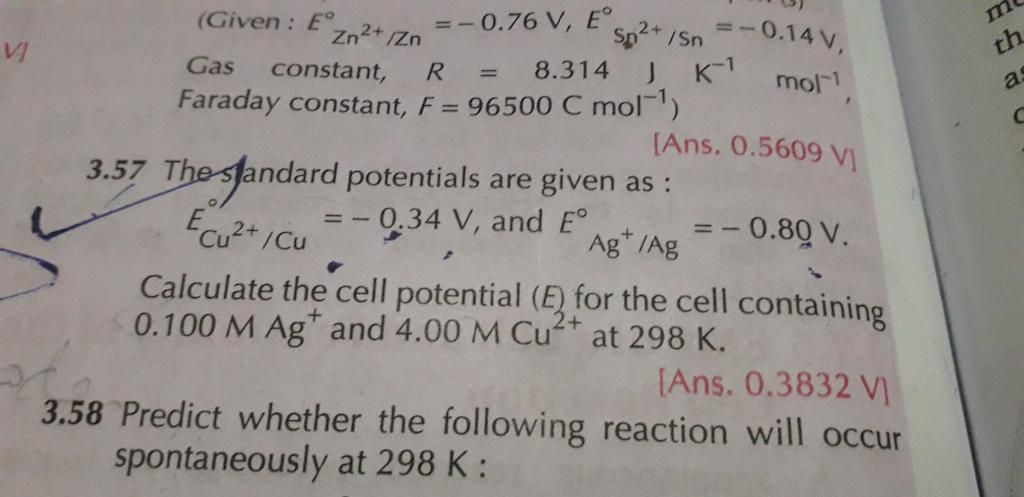CBSE Class 12-science Answered
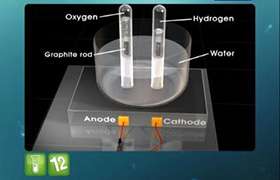
An electric current was passed through two voltameter cells one containing copper sulphate (using copper electrodes) and the other containing silver nitrate solution (using silver electrodes). The increase in weights of cathode in two cases was respectively 0.189 gm and 0.648 gm. Calculate the chemical equivalent of copper, taking that of silver as 108.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
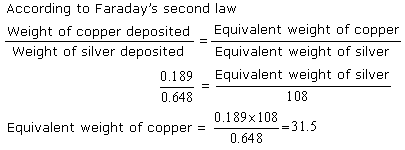
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 05:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 04:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 08:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 09:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:11: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Jul, 2019, 09:49: PM