CBSE Class 11-science Answered
An electric bulb of volume 250 cm3 was sealed off during manufacture at the pressure of 10-3 mm of mercury at 27oC. Find the number of air molecules in the bulb.
Asked by Topperlearning User | 08 May, 2015, 11:19: AM
Let n be the number of air molecules in the bulb.
Given : V1 = 250 cm3, P1 = 10-3 mm of Hg, T1 = 300 K
Using PV = nRT, where R is the universal gas constant, we get
10-3 x 250 = nR x 300 ...................(i)
At N.T.P, one molecule of air occupies a volume of 22400 cm3. So, at N.T.P,
V2 = 22400 cm3, P2 = 760 mm of Hg, T2 = 273 K, n2 = N = 6 x 1023 molecules
Therefore, 760 x 22400 = 6 x 1023 x R x 273 ......................(ii)
Dividing (i) by (ii), we get
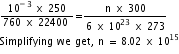
Answered by | 08 May, 2015, 01:19: PM
Concept Videos
CBSE 11-science - Physics
Asked by ifrayaseen31 | 28 Oct, 2023, 09:26: AM
CBSE 11-science - Physics
Asked by karanchandra34 | 29 Jan, 2019, 11:10: PM
CBSE 11-science - Physics
Asked by Madhurimaurya609 | 11 Jul, 2018, 08:14: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM