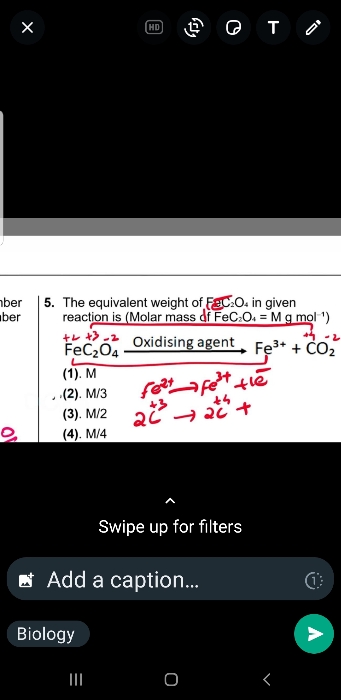
NEET Class neet Answered
an ecess of AgNO3 is added to 100 ml of a 0.01 M solution of dichlorotetraaquachromium (III) chloride. the number of mole of AgCl precipited would be

Asked by nitinkawadkar | 26 Apr, 2019, 07:15: AM
Given:
Molarity of solution of dichlorotetraaquachromium (III) chloride = 0.01 M
Volume of solution of dichlorotetraaquachromium (III) chloride = 100 ml
The formula of dichlorotetraaquachromium (III) chloride is [Cr(H2O)Cl2]Cl
On ionisation,
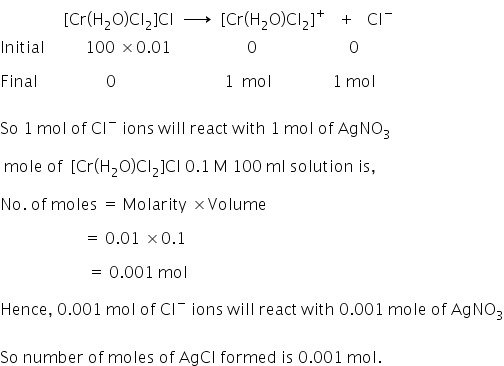
Moles of AgCl formed is 0.001 mol
Answered by Varsha | 26 Apr, 2019, 12:13: PM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM
NEET neet - Chemistry
Asked by cherishchoudhary555 | 17 Jul, 2022, 10:28: PM