CBSE Class 11-science Answered
Among the following structures of SF4 which one is more stable and why? What is this shape called? 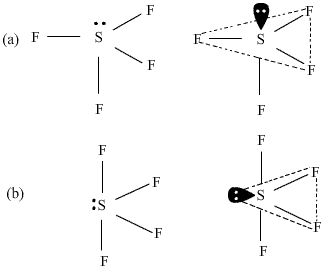

Asked by Topperlearning User | 13 Jun, 2016, 02:40: PM
In figure (a), the lone pair of electrons is present at the axial position so there are three lone pair-bond pair repulsions at 90o. In the figure(b), the lone pair of electrons is present at the equatorial position and there are two lone pair- bond pair repulsions. Hence figure (b) is more stable as compared to figure (a). The shape shown in figure (b) is distorted tetrahedron, a folded square or a see saw.
Answered by | 13 Jun, 2016, 04:40: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by Amit176039 | 03 Oct, 2020, 03:21: PM
CBSE 11-science - Chemistry
Asked by shubhamanand1369 | 26 May, 2020, 11:38: AM
CBSE 11-science - Chemistry
Asked by amangeneralstore27 | 21 Dec, 2019, 11:25: AM
CBSE 11-science - Chemistry
Asked by atulpd | 29 Mar, 2018, 10:42: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 03:00: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 03:01: PM