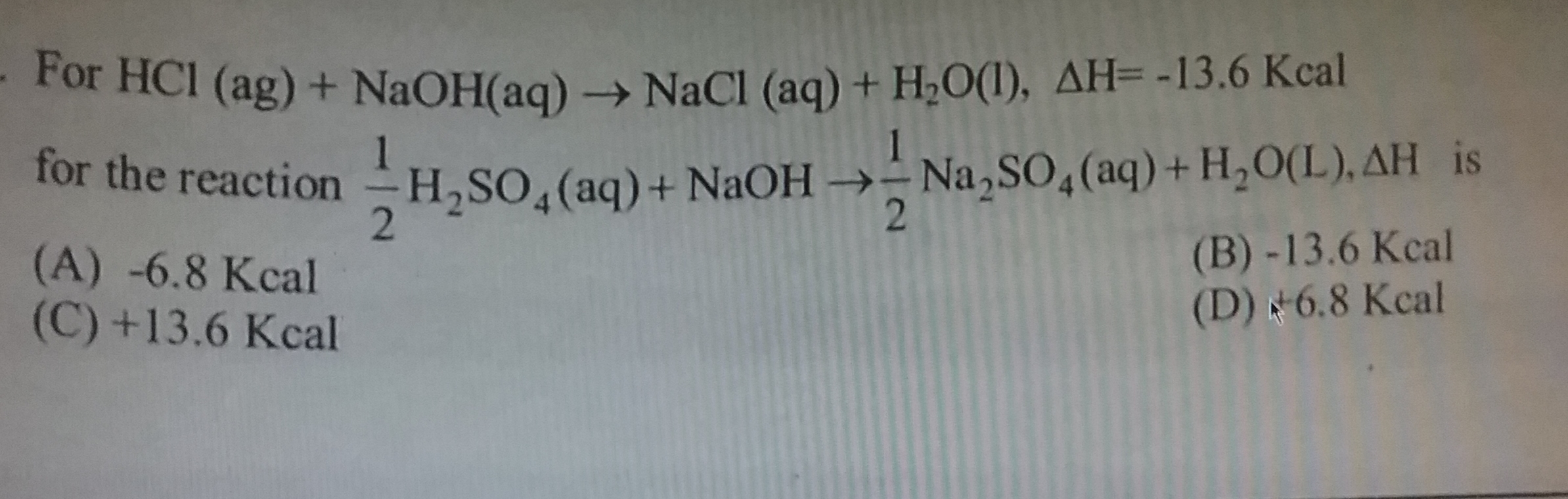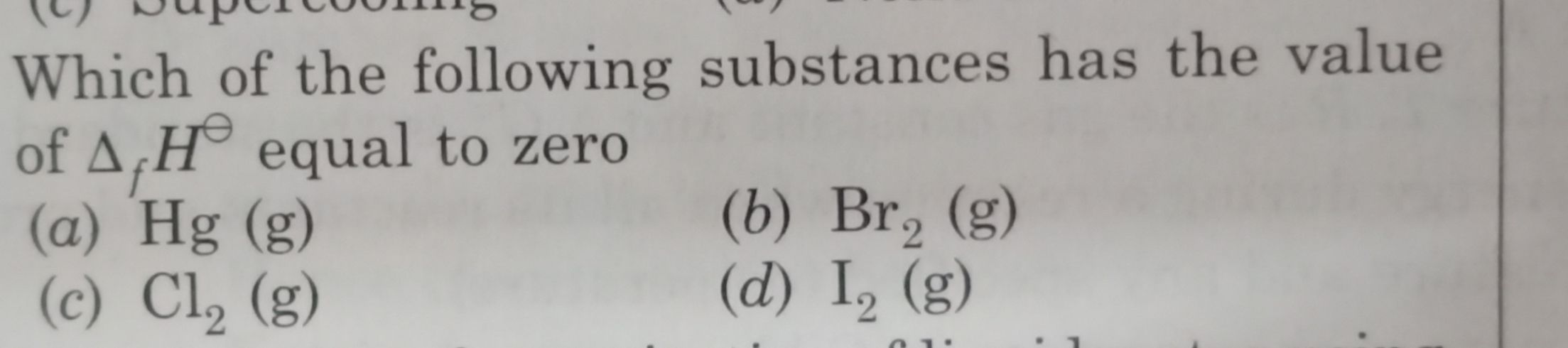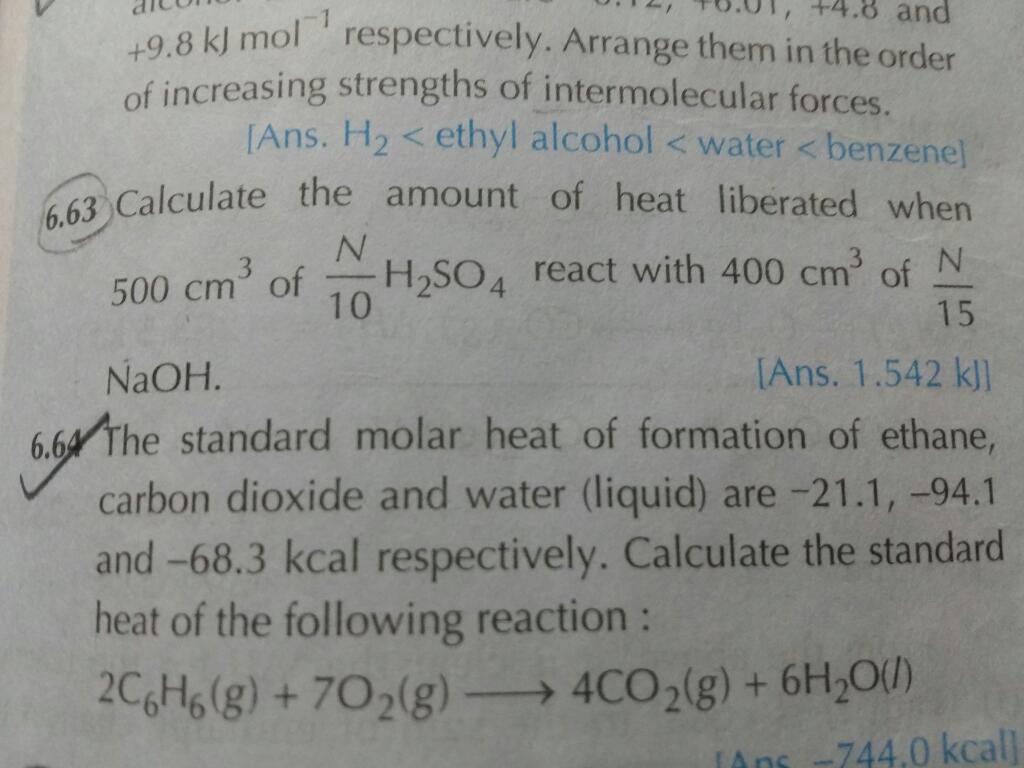CBSE Class 11-science Answered
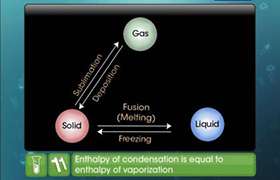
Among the enthalpies of fusion and enthalpies of freezing, which one is positive and which one is negative and why?
Asked by Topperlearning User | 15 Jun, 2016, 05:39: PM
Melting is endothermic process, so all enthalpies of fusion are positive because ice requires heat for melting. While in freezing of water equal amount of heat is given to surrounding i.e. freezing is exothermic process. Thus all enthalpies of freezing are negative.
Answered by | 15 Jun, 2016, 07:39: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM