ICSE Class 10 Answered
About 40 g of sulphur is taken in an enclosed vessel containing 22.4 litres of oxygen. The sulphur is ignited. Calculate the volume of sulphur dioxide formed, and the mass of uncombined sulphur.
(1 mole of S = 32g: molar volume = 22.4 litres)
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
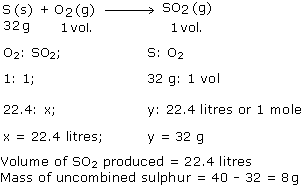
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 05 Mar, 2024, 06:40: PM
ICSE 10 - Chemistry
Asked by kundus458 | 07 Feb, 2024, 08:55: AM
ICSE 10 - Chemistry
Asked by matloobser | 07 Sep, 2023, 09:36: AM
ICSE 10 - Chemistry
Asked by dafk04.dp | 06 May, 2021, 06:22: PM
ICSE 10 - Chemistry
Asked by amit.clw4 | 15 Mar, 2021, 07:27: AM
ICSE 10 - Chemistry
Asked by amit.clw4 | 14 Mar, 2021, 08:12: AM
ICSE 10 - Chemistry
Asked by ravi.solaabhi | 17 Oct, 2020, 10:11: AM
ICSE 10 - Chemistry
Asked by payalagrawal1724 | 28 Jun, 2020, 07:22: PM
ICSE 10 - Chemistry
Asked by vijay.prag | 29 Dec, 2019, 08:07: PM
ICSE 10 - Chemistry
Asked by Dsangayy | 08 May, 2019, 07:11: PM






