CBSE Class 9 Answered
(a) Atomic number = 11
Electronic configuration = 2, 8, 1
Atomic number = 19
Electronic configuration = 2, 8, 8, 1
(b) The following are the observations of Rutherford's α-particle scattering experiment:
(i) Most of the α-particles passed straight through the gold foil.
(ii) Some of the α-particles were deflected by the foil by small angles.
(iii) Surprisingly, one out of every 12,000 particles appeared to rebound.
OR
(a)
|
Isotopes |
Isobars |
|
1. Atoms of the same element having same atomic number but different mass number. |
1. Atoms of the different elements having different atomic number but same mass number. |
|
2. They have same chemical properties but different physical properties . |
2. They possess different physical and chemical properties. |
|
3. Examples : |
3. Examples : |
(b) Maximum number of electrons in K shell = 2n2
For K shell, n = 1
Maximum number of electrons in K shell = 2
(c) Atoms combine with other atoms to achieve the electronic configuration of nearest noble gas and become more stable.

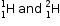 .
.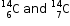 .
.







