CBSE Class 9 Answered
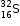 .
(d) State the difference between a proton and an electron on the basis of their location.
(e) Give one use of an isotope of cobalt and one use of an isotope of iodine.
OR
(a) During radioactive disintegration, an atom gets converted into another atom whose mass number remains the same but atomic number increases by one. Will the new atom formed be an isobar or isotope of the parent atom?
(b) Which is heavier: a proton or an electron and how many times?
(c) How many electrons are present in the outer shell of the atom of a noble gas other than helium?
(d) Write the drawbacks of Rutherford's model.
.
(d) State the difference between a proton and an electron on the basis of their location.
(e) Give one use of an isotope of cobalt and one use of an isotope of iodine.
OR
(a) During radioactive disintegration, an atom gets converted into another atom whose mass number remains the same but atomic number increases by one. Will the new atom formed be an isobar or isotope of the parent atom?
(b) Which is heavier: a proton or an electron and how many times?
(c) How many electrons are present in the outer shell of the atom of a noble gas other than helium?
(d) Write the drawbacks of Rutherford's model.(a) Neon and argon have completely filled electrons in their outermost shell with eight electrons and cannot lose, gain or share electrons with atoms of other elements. Therefore, the valency of these gases is zero.
(b) Electronic configuration of sodium = 2, 8, 1
Therefore, its valency is one.
Electronic configuration of oxygen = 2, 6
Therefore, its valency is two.
(c) Atomic structure of sulphur
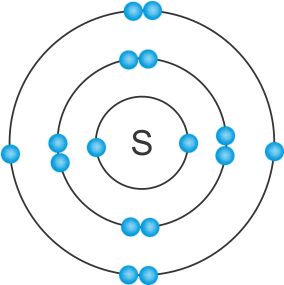
(d) A proton is located within the nucleus of an atom while an electron revolves in the orbits around the nucleus of an atom.
(e) An isotope of cobalt is used in treatment of cancer.
An isotope of iodine is used in treatment of goiter.
OR
(a) The new atom formed will be an isobar since isobars have same mass number but different atomic number.
(b) A proton is heavier than electron. The mass of a proton is 1837 times than that of an electron.
(c) Eight
(d) The drawback of Rutherford's model of atomic structure is about the stability of revolving electrons. When a body revolves in an orbit, it always undergoes acceleration due to change in direction. So, the revolving charged electrons should emit energy in the form of radiation. This loss in energy will shrink the orbit and ultimately electron will hit the nucleus and thus atom will not be stable.









