ICSE Class 10 Answered
(a) Solution ‘P’ has p H of 13, solution Q has a p H of 6 and solution R has a p H of 2.
(i)Which solution will liberate Ammonia from Ammonium sulphate?
(ii)Which solution contains solute molecules as well as ions?
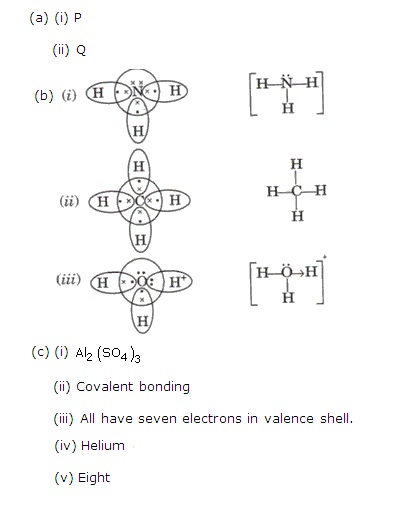
(b)Give the electron dot structure of the following:
(i)NH3
(ii)CH4
(iii)H3O+
(c)(i) Write the fornula of the sulphate of the element with atomic number-13.
(ii)What type of bonding will be present in the oxide of the element with atomic number 1?
(iii)Which feature of the atomic structure accounts for the similarities in the chemical properties of the elements in group 17 of the periodic table?
(iv)Name the element which has highest ionization potential.
(v)How many electrons are present in the valence shell of the element with atomic number 18?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
ICSE 10 - Chemistry
Asked by chatterjee.chaitalichat | 03 Feb, 2020, 12:12: AM





